By Alexander Ekemenah, Chief Analyst, NEXTMONEY
Introduction
The Oxford/AstraZeneca vaccine has no doubt run into the murky and shark-infested water of European Union geopolitics within the context of the EU-Brexit controversy of pre-COVID-19 era. In other words, Oxford/AstraZeneca vaccine rejection by some European Union countries stemmed out of the unfinished business of Brexit and the vindictive wish to punish the United Kingdom for its peacock arrogance and brash exit from the European Union in which Britain is seen as an outlaw or outcast from the mainstream European continental politics.
Thus the EU geopolitical rejection of the Oxford/AstraZeneca vaccine by several European countries is like a vicious blow to the solar plexus of the British ruling class and its insufferable arrogance. The British did not see it coming at all on the horizon. Why it is more serious, in strategic sense, is because Oxford/AstraZeneca vaccine is the only major vaccine coming out of the United Kingdom, at least for now. Thus the rejection is not just a reputational damage to the Oxford/AstraZeneca vaccine brandname but also to the Brits’ pride of place and awareness of its vulgarized position in world affairs. It is a high reputational damage to its diplomatic prowess as the Brits can be seen running from pillar to pole to do damage control. British diplomacy failed this time around to stem the tidal wave of geopolitical tornado or hurricane coming across the English Channel from the main heartland of the European continent. It tells that the Brits may wish to go their own way and the rest of Europe may not care about this. There is an African proverb that says “when a stream decides to leave or cut off from its source, it must be ready to face the consequences of being abandoned to whatever fate it subsequently encounters on its own journey”!
As a result of this snafu, AstraZeneca’s shares on London Stock Exchange fell by 1.5 per cent as far back as late November 2020 even before the crisis snowballed into the huge controversy that it is by mid-March 2021. This is a huge financial loss in an economy that had earlier been battered by the coronavirus pandemic. It is a double jeopardy and major setback for the British bourgeoisie at a time when the world is trying to recover from the negative effects of the coronavirus pandemic. So much goodwill, hope and confidence have been invested in the production of the vaccine – only for all these to be shattered right before everybody’s eyes.
But interestingly while all eyes are fixed on the United Kingdom, AstraZeneca is an Anglo-Swedish pharmaceutical giant. Eyes are inadventently diverted from the role of Sweden in the controversy. Sweden was another sick “man” of Europe during the raging pandemic. Sweden for a long time refused to order lockdown and impose other safety measures in preference for what it called “herd immunity” which at the end of 2020 have seen thousands of Swedes dying like flies. Equally interesting is the fact that Sweden was one of the countries that have so far rejected the Oxford/AstraZeneca vaccine in a manner akin to Pontius Pilate washing off his hands in denial of whatever sophistries the Brits are putting up in defense of the vaccine!
The only saving grace for the Oxford/AstraZeneca vaccine has been the developing countries, most especially the former British colonies like Nigeria, countries that can be argued not to care about the explosive controversy because of their legacy hangover of colonial mentality and because of their narrow selfish interests involved with the importation of the vaccine and the concomitant pecuniary gains involved.
In addition to the overall picturesque of this crisis and mess is the involvement and role of University of Oxford which may never be understood, probably for some time to come. Unversity of Oxford is no doubt one of the topmost universities in the world that has earned accolades for its advance researches and intelletual/academic prowess that have push back the boundary of pedestrian ignorance in the world. A team of intrepid Oxford scientists is the originator of the controversial vaccine while AstraZeneca is the manufacturer.
Behind the public scene the British ruling political class is baffled by the sudden turn of unsavoury events in which the Oxford/AstraZeneca is at the epicentre which in turn has led to the United Kingdom being kicked around like football by the EU politics of mortar-and-pestle. United Kingdom is being snubbed left, right, and centre in a way that has not happened probably since the Second World War. United Kingdom is being kicked in the ass, slapped on both cheeks, knocked viciously on the head with a cudgel forcing it to run like a wounded unicorn in the wild!
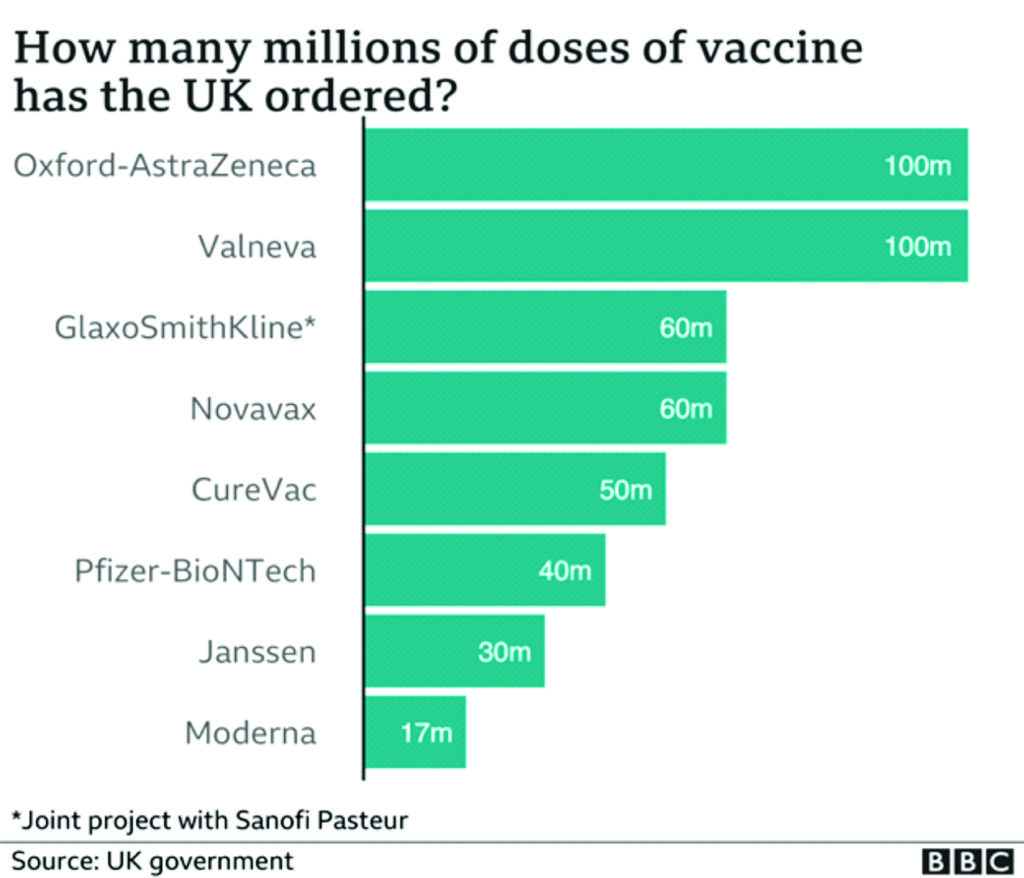
The world is currently scrambling and stampeding for the available vaccines on the gobal market shelves. With what has happened to Oxford/AstraZeneca vaccine, it is highly probable that more revelations will come to the public domain in several other countries on how governments have handled or are handling the coronavirus pandemic and its fallouts. Coronavirus pandemic will generate more controversies than expected in the coming years. The Oxford/AstraZeneca vaccine controversy is just the tip of the iceberg about the shenaniganisms of governments and private sector operators that have taken place unbeknown to the public. Much will be swept under the carpet. But much will also be revealed to the astonishment of the public and great embarrassment and shame of the main actors whether government officials or business executives.
This article seeks to disambiguate the controversy faced by Oxford/AstraZeneca vaccine over the double results obtained while testing for its efficacy.
A Kick to the Groin
The hurricane against the Oxford/AstraZeneca vaccine came as a gradual build-up into the tsunamic public relations disaster it has become for all the parties concerned: University of Oxford, AstraZeneca company, the British Government, other stakeholders and even the British public.
AstraZeneca’s COVID-19 vaccine rollout has gotten off to a rocky start in Europe—to put it mildly. First, a supply shortfall triggered a public back-and-forth between executives and government officials. Then several countries expressed doubts about how well the vaccine works in people over 65. Now seven countries are raising safety concerns.1 Denmark, Norway, Austria, Estonia, Latvia, Lithuania and Luxembourg have halted some or all of their AstraZeneca COVID-19 vaccinations over fears of blood clots, France24 reports. Previously, Austria had stopped using a single batch of the vaccine after a clotting issue turned up in one recipient. In the wake of the news, Estonia, Latvia, Lithuania and Luxembourg stopped using vaccines from the same batch, France24 reports. Denmark and Norway temporarily stopped all vaccinations with AZ shots, according to the report.2
An AstraZeneca spokesperson said patient safety is the company’s “highest priority.” “Regulators have clear and stringent efficacy and safety standards for the approval of any new medicine, and that includes COVID-19 Vaccine AstraZeneca,” she said. “The safety of the vaccine has been extensively studied in Phase III clinical trials and peer-reviewed data confirms the vaccine has been generally well tolerated.”3
Thursday’s news is only the latest negative development for the rollout of AZ’s product. Since the vaccine’s debut in Europe, public comments from governments, officials and even doctors have raised questions about the vaccine. Germany restricted its use in people 65 and older, citing a lack of data in the age group, and then reversed course. When the shot was initially restricted in her age group, German chancellor Angela Merkel said she doesn’t “belong to the group recommended for AstraZeneca.” Some publications ran with headlines that she’d refused the shot, creating confusion for citizens.4
In January, French President Emmanuel Macron said the vaccine was “quasi-ineffective” in people 65 and older before his country gave the green light in the age group. Adding to all the confusion, thousands of healthcare workers in Europe have refused the shot, Forbes reported last month [February]. They argued they should receive the mRNA vaccines from Pfizer/BioNTech or Moderna.5
The result? Europe’s vaccination program is lagging in other developed countries, as many AstraZeneca shots go unused. As of Thursday, France, Germany, Italy and Poland had used less than half of the AstraZeneca vaccine doses on stock, according to data from the European Centre for Disease Prevention and Control. Those countries have received the highest number of doses, followed by Spain, which has used around 60% of its available stock and is pressing ahead with vaccinations.6
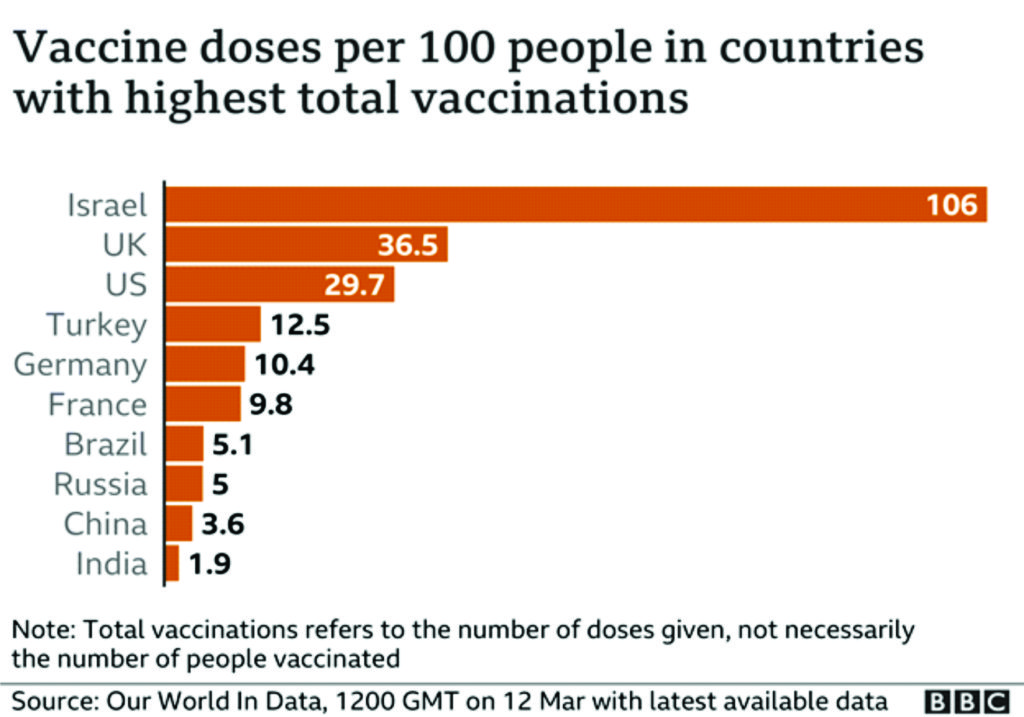
Meanwhile, Europe significantly lags Israel, the UAE, the U.K., Chile and the U.S. in vaccinations given per 100 people, according to Our World In Data.7
[As at March 15, 2021], Germany, France, Spain, Italy, Ireland and the Netherlands have joined the growing list of countries that have suspended the use of the coronavirus vaccine developed by AstraZeneca and the University of Oxford over blood clot concerns.8 The Dutch government said Sunday that the Oxford-AstraZeneca vaccine would not be used until at least March 29, while Ireland said earlier in the day that it had temporarily suspended the shot as a precautionary step.9 On Monday, the German government also said it was suspending its use, with the vaccine regulator, the Paul Ehrlich Institute, calling for further investigations. The Italian medicines authority made a similar announcement on Monday afternoon and French President Emmanuel Macron also said the vaccine’s use would be paused pending a verdict from the EU’s regulator. Spain Health Minister Carolina Darias said Monday that the country will halt use of the shot for at least two weeks, Reuters reported, and Portugal and Slovenia also suspended the vaccine.10
[But] the World Health Organization has sought to downplay ongoing safety concerns, saying last week that there is no link between the shot and an increased risk of developing blood clots. The United Nations health agency has urged nations to continue using the Oxford-AstraZeneca vaccine.11 Despite this, a number of European countries have already paused the use of the Oxford-AstraZeneca vaccine. It has added to the woes of the region’s ailing vaccination campaign at a time when Germany’s public health agency has warned that a third wave infections has already begun.12 Thailand [far away from continental Europe] has also halted its planned deployment of the vaccine.13
The move to pause its use by Dutch and Irish officials came shortly after Norway’s medicines agency said it had been notified of three health workers being treated in hospital for bleeding, blood clots and a low count of blood platelets after receiving the Oxford-AstraZeneca vaccine. Norway has put its Oxford-AstraZeneca vaccine program on hold.14 Geir Bukholm, director of the division of infection control and environmental health at the Norwegian Institute of Public Health, said Norway’s medicines agency would “follow up on these suspected side effects and take the necessary measures in this serious situation.”15
Europe’s drug regulator, the European Medicines Agency, has said there is no indication that the Oxford-AstraZeneca vaccine is causing blood clots, adding that it believes the vaccine’s benefits “continue to outweigh its risks.” The EMA acknowledged some European countries had paused the use of the Oxford-AstraZeneca shot but said inoculations may continue to be administered while an investigation of blood clot cases is ongoing.16
Irish Prime Minister Micheal Martin told CNBC on Monday that he anticipated the pause would be temporary and the country was aiming to catch up quickly with its inoculation program. “There is no causal effect established or anything like that yet, but as a precautionary move in line with the precautionary principle and in an abundance of caution, our clinical advice was to pause the program whilst the EMA does a review of this,” he said. “This is an unwelcome pause but nevertheless I think it’s important that we take heed of the advice we have received.”17
In far-away Thailand, another hurricane was brewing.
Thai Prime Minister Prayuth Chan-ocha became the first person to be inoculated with the AstraZeneca Covid-19 vaccine in the Southeast Asian country on Tuesday after the rollout had been temporarily put on hold over safety concerns.18
Prayuth and other cabinet members had been initially due to get their vaccine shots on Friday, before Thailand suspended the use of the AstraZeneca vaccine after reports it could cause blood clots prompted a number of European countries to hit pause. “Today I’m boosting confidence for the general public,” Prayuth told reporters at Government House, before he received a shot in his left arm. Prayuth, who will turn 67 this month, later said he felt fine after the injection. Thailand’s health minister said on Monday the rollout would resume after many countries had said there were no blood clot issues with the vaccine.19
Thailand has started vaccinating frontline health workers and other groups including government officials using imported shots but the country’s overall vaccination strategy is heavily reliant on making the AstraZeneca vaccine domestically.20
The AstraZeneca vaccine is due to be produced by a company owned by the country’s king, with 61 million doses reserved for the country’s population. The Thai-produced AstraZeneca vaccine is not expected to be available until at least June, when Thailand plans to begin its mass inoculation campaign. Prayuth and his cabinet were injected with some of the 117,300 doses of imported AstraZeneca vaccine Thailand received for emergency use earlier this month.21
Thailand previously imported 200,000 doses of Sinovac’s CoronaVac. A further 800,000 CoronaVac doses would arrive later this month, followed by a million more in April.22
All these did not prevent Thailand from also suspending or pausing the use of the Oxford/AstraZeneca vaccine alongside other European countries. The EU geopolitic has ostensibly catch up with Thailand from which it could not escape its entrapment.
The rejection of the vaccine is continental-wide, from Benelux, Nordic to Central European countries. The “Big Boys”: Germany, France, Italy, Spain including Austria joined the “riotous” crowd of “smaller countries” to reject the vaccine. Nothing like this has ever happened in recent memory. Something is unarguably fundamentally wrong somewhere. Britain did not ostensibly bargain for this poor reception and rejection of a notable pharmaceutical drug, a virus vaccine in this case, at this point in time. It came out of the dark cloud, unplanned for!
How the Oxford/AstraZeneca controversy blew open
The rejection of the Oxford/AstraZeneca vaccine came like a thief in the night. Apparently in the day, the University and the pharmaceutical giant have committed blunder that would brought the thief into the household in the night. There is no effect without a cause or vice versa. The duo can be seen to have been negligent of certain details and procedures that would collectively come to cost them their reputation and enotmous financial loss. The event that started as honey would turn out to be vinegar.
The problem started with was initially perceived to be that of demand-and-supply disequilibria.
After months of research and a week-long controversy over a reduction in expected vaccine supply, regulators in Europe have authorized AstraZeneca’s two-dose COVID-19 vaccine.23 The vaccine, the result of AZ’s collaboration with Oxford University, is conditionally approved across Europe in people ages 18 and older. A broad rollout in EU member countries will follow, but in recent days, a reduction in first-quarter supply has dominated headlines.24
Last week [i.e. early February 2021], AstraZeneca notified EU officials of manufacturing problems in its supply chain that would cause it to drastically cut first-quarter shipments to the continent. An intense back-and-forth [negotiation] followed, and on Friday, Europe restricted exports of COVID-19 vaccines.25
On a call with reporters Friday, AstraZeneca CEO Pascal Soriot said that manufacturing processes typically take years to develop and refine. During the pandemic, the company and its production partners have set up the manufacturing process in the span of “a few months.” The result is that millions of doses will be available sooner, he said, but still the process hasn’t been fully refined at all of the sites. Some sites are farther along in the “learning curve,” he said, so AZ is seeing a “variability of yield” throughout different sites in its network. The company has had a “good discussion” with Europe and is focused on improving supply, he said.26
The AstraZeneca authorization follows similar green lights from European officials for mRNA vaccines from Pfizer/BioNTech and Moderna. But while those vaccine regimens require doses to be given several weeks apart, AZ’s shots can be administered between 4 and 12 weeks part, providing for greater flexibility, Soriot noted. That means if AZ ships an initial 20 million doses, European health officials could use all of them to start to vaccinate 20 million people, he said. Then, up to three months later, those recipients could get their second shots from another shipment.27
It would soon turn out that the demand-and-supply disequilibra is not the only problem plaguing the Oxford/AstraZeneca vaccine. More potent is the two-dose connundrum that has been lurking in the backing until it blew open to the public domain. This was to become more problematic than imagined by the forces behind the vaccine production and roll-out. It all started with several countries expressing doubts about the efficacy of the vaccine in people over 65 years of age when tests revealed two different sets of result for people of two different age group.
In its phase 3 trial, AstraZeneca’s vaccine was 70% effective overall, but a dosing error yielded a higher result in a subset of participants. For people who received a half dose followed by a full dose, vaccine efficacy was 90%. The trial results prompted AZ to run another study for a potential emergency use authorization in the U.S. That study is fully enrolled and set to read out next month [February 2021], AZ execs said on Friday’s call.28
AZ’s authorization came right after COVID-19 vaccines from Novavax and Johnson&Jonson posted their own phase 3 data. Soriot called all of the developments “positive news for the entire world.”29
Pascal Soriot could now be seen to be gravely mistaken in his euphoria. While the world might have been celebrating the arrival of various vaccines into the global market shelf and the subsequent rolling out of the vaccination exercise, the Oxford/AstraZeneca vaccine of which he is the main supervisor hit a brickwall across the English Channel in the continental Europe. Ostensibly, Soriot did not, in his wildest imagination, bargain for the nasty reception of the vaccine across the English Channel. He did not see it coming at all. Even when the error in dosing was discovered, he still could not expect that European Union would develop cold feets and outrightly ban the vaccine from being administered to citizens across Europe.
The dosing error would become a Frankenstein monster in the boardroom of AstraZeneca and University of Oxford threatening to scatter and undo all the previous investment in the vaccine. It became the strategic faux-pax for which the EU is now threatening to hang or guillotine the vaccine by the roadside for all to see the ugly spectacle of a public execution.
There is no doubt that these were some of the same countries that performed poorly, that were practically seen to be helpless when the pandemic was raging and ravaging their countries, plucking off their citizens in thousands and sending them to the Greater Beyond. But when suddenly some couple of people died with or without causal connection to the Oxford/AstraZeneca vaccine they suddenly became holier-than-thou, seemingly hitting vengefully back at Britain where it is most painful, giving it a bloody face with a heavy punch to the nose. It is analogous to a street brawl where a lone individual was given a beating of his life by a gang of bad boys.
How University of Oxford found itself in this ugly and messy situation may probably never be understood. But there can be no denial of the Oxford’s good intention about its foray into the scientific and medical search for the vaccine solution to the raging pandemic. Its venture is salutory. Its collaboration with AstraZeneca may not also be questioned on the basis of this good volitional intention to find vaccine for the coronavirus pandemic. In short, University of Oxford stands on unquestionable high philosophical, scientific, medical and even political pedestals as regard its involvement and engagement with AstraZeneca to produce the AZD1222 vaccine for the British and world markets.
However, Oxford/AstraZeneca found itself in a hyper-competitive environment among the world top-most Big Pharma vaccine makers including those sponsored directly by Governments, for instance, in Russia and China. It is a clustered global environment only meant for the “Big Boys” in the pharmaceutical industry. Every company engaged and working to produce the first vaccine for the coronavirus pandemic find itself working under extreme pressure in a VUCAed environment of vulnerability, uncertainty, complexity and ambiguity that have also come to affect and pervade the global scientific community to a large degree like the geopolitical environment. It is a rat- or horse-race to the top of the ladder!
Its sins may probably be found in the manner it approached the enterprise: the procedures adopted, the pressure mode under which its scientists forced themselves to work in the race for the vaccine knowing fully the dangers involved in such conditions. Under such pressurized and/or heated environment, mistake can easily occur. Under this perceptible unbearable conditions, a slip or slight mistake becomes costly in its composite effects. And that was exactly what happened.
Thus, in passing the final judgment, University of Oxford may not be accused of wilful intent to cause harm to any of the end-users of the AZD1222 vaccine – for instance, all those who are claimed to have died as a result of blood clot linked to the vaccine. By extension too, AstraZeneca may not also be accused of such “evil” intention at all. What happened was a combination of avoidable “errors” that conspired against the perceptible good volition to serve and save humanity from the skulduggery and yoke of the coronavirus pandemic.
However, the error, mistake, snafu (several adjectives have been deployed to describe what has happened – depending on the sentiment of the individual writer) that has come to lead to international controversy dates back to 2020. The Oxford/AstraZeneca vaccine problem started late 2020 precisely when it was announced as a “breaking news” which sent the British populace into wild jubilation that would later turn awry and sour in the mouth by March 2021. A back-review reveals that something momentous has gone wrong in the process of producing and testing the efficacy of the vaccine.
A good account of what transpired during the experimental stage of the vaccine was provided by Fergus Walsh reporting for BBC News.30 It is a story of intrepid scientists (such as Prof Teresa Lambe, Prof Sarah Gilbert, and Prof Andrew Pollard) working round the clock to find the template for the new experimental coronavirus vaccine. Interestingly, this was in January 2020 when the coronavirus from China has not even been declared a pandemic by any official quarter. These scientists may have been guided by intuition, whether they are aware of this or not, to embark on this journey that brought the whole British society to where it is today. The scientists were looking for the Weapon X to fight what was coming on the horizon not knowing exactly what this enemy look like. The Weapon X is what turned out to be the Oxford/AstraZeneca (AZD1222) vaccine they had agreed upon to be the stealth or visible fighter against the coronavirus.
In the early hours of Saturday 11 January, according to Fergus Walsh in his report, Prof Teresa Lambe was woken up by the ping of her email. The information she had been waiting for had just arrived in her inbox: the genetic code for a new coronavirus, shared worldwide by scientists in China. She got to work straight away, still in her pyjamas, and was glued to her laptop for the next 48 hours. “My family didn’t see me very much that weekend, but I think that set the tone for the rest of the year,” she says. By Monday morning, she had it: the template for a new experimental coronavirus vaccine. The first death from the new virus was reported around the same time, but it was still a month before the disease it causes was named Covid-19.31
Lambe’s team at Oxford University’s Jenner Institute, led by Prof Sarah Gilbert, was always on the lookout for Disease X – the name given to the unknown infectious agent that could trigger the next pandemic. They had already used their experimental vaccine system against malaria and flu and, crucially, against another type of coronavirus, Middle Eastern Respiratory Syndrome (Mers). So they were confident it could work again.32
That weekend was the first step on a journey to create a vaccine at lightning speed, for a disease that would, in a matter of months, claim more than 1.5 million lives. … There have been dramas along the way, including:
- a rush to charter a jet when a flight-ban prevented vaccine from getting into the country
- dismay at totally false reports on social media that the first volunteer to be immunised had died
- concern that falling infection rates over the summer would jeopardise the hope of quick results
- how an initial half-dose of the vaccine unexpectedly provided the best protection
- an admission from the chief of Oxford’s partner, drug company AstraZeneca, that it would have run the trials “a bit differently”.
In the middle of January Prof Andrew Pollard, the director of the Oxford Vaccine Group, which runs clinical trials, shared a taxi with a modeller who worked for the UK government’s Scientific Advisory Group for Emergencies. During the journey, the scientist told him data suggested there was going to be a pandemic not unlike the 1918 flu. “I went from someone who was aware of a small outbreak in China, which was of academic interest, to realising that it was going to change our lives. It was a chilling moment,” Pollard says.33
Trouble, however, seemed to start during the trial, i.e. testing the efficacy of the vaccine which was found to be satisfactory, without side-effects, up to a point. The green light to go ahead wth the mass production of the vaccine was already flashing on the dashboard.
As the trial grew, it was clear that Oxford’s small manufacturing facility would not be able to keep up with demand. The team decided to outsource some of the manufacturing to Italy. But when the first batch was ready, there was a snag – the Europe-wide lockdown meant there were no flights to airlift it from Rome. “Eventually we chartered a plane to bring 500 doses of vaccine because it was the only way we could get it here in time,” says Green.34
This is a really important part of the story which ended up being highly significant months later.35
The Italian manufacturers used a different technique to Oxford to check the concentration of the vaccine – effectively how many viral particles are floating in each dose. When the Oxford scientists used their method, it appeared that the Italian vaccine was double strength. What to do? Calls were made to the medical regulators. It was agreed that volunteers should be given a half measure of the vaccine, on the basis that it was likely to equate to something more like a regular dose. This was partly a safety issue – they preferred to give them too little rather than too much.36
But after a week, the scientists became aware that something unusual was going on. The volunteers were getting none of the usual side-effects – such as sore arms or fever. About 1,300 volunteers had only received a half-dose of the vaccine, rather than a full one. The independent regulators said the trial should continue and that the half-dose group could remain in the study.37
The Oxford team bristle at any suggestion that there was a mistake, error, call it what you will. Perhaps the most accurate characterisation is that the volunteers were inadvertently given a lower dose. In months to come, they would be the stellar group in terms of vaccine efficacy.38
From the start, the team at Oxford had had the goal of creating a vaccine that could help the world. To do that they would need billions of doses – something only industry could provide. Sir Mene Pangalos, of the Cambridge-based pharmaceutical giant AstraZeneca, suggested the company could help.39
But there was a potential hitch – Oxford insisted the vaccine should be affordable, which meant no profit for the pharmaceutical company. “Not usually the way big pharma works,” says Pollard.40
After intense negotiations, they reached a deal at the end of April. The vaccine would be provided on a not-for-profit basis worldwide, for the duration of the pandemic, and always at cost to low- and middle-income countries. Most importantly of all, AstraZeneca agreed to take on the financial risk, even if the vaccine turned out not to work. In May, in a huge vote of confidence, the UK government agreed to buy 100 million doses and provided nearly £90m in support. By then there were nearly a dozen coronavirus vaccines in human trials around the world.41
On 20 July, the initial results were released, showing whether the vaccine had stimulated the immune systems of the first 1,000 volunteers. The team was feeling the pressure. “I’ve worked in vaccines for long enough to know that most vaccines actually don’t work. And it’s the worst feeling in the world to have such high expectations and then to see nothing,” says Prof Katie Ewer, who leads the team studying T-cell response.42
It was good news, though. The vaccine appeared to be safe, and had triggered the two-pronged immune response they were hoping for – producing antibodies which neutralise the virus, and T cells which can kill cells which have become infected. But the data also led to a change of strategy. Until then, the Oxford team had hoped to produce a vaccine that could be delivered in a single dose, so there was more vaccine to go round.43
However, 10 participants who were given two doses showed a much stronger immune response, so all volunteers were invited back for a booster, to maximise the chances of protection. Although these early results were promising, at this stage there was still no evidence to show whether the vaccine would actually protect against Covid-19.44
Intriguingly, when the trial results came out, there were indications it may partly suppress transmission of the virus. But more evidence is needed. By the end of the summer, trials of the vaccine were running in six countries including the UK, Brazil, South Africa and the United States. Nearly 20,000 volunteers had been recruited. But on 6 September suddenly everything stopped. A participant had developed a rare neurological condition. “In a clinical trial of tens of thousands of people, stuff happens,” says Pollard. “People develop illnesses, there will be people who develop cancers and who develop neurological conditions – the difficulty… is to work out whether it’s associated with the… vaccine.45
An independent safety review did not find any reason to suspect the illness had been caused by the vaccine. But they couldn’t rule it out either. The volunteer is still part of the trial and is recovering. Safety regulators in the UK, Brazil and South Africa gave the green light for the trials to resume within days, but it was six weeks before they were allowed to resume in the US. All this came amidst a climate of anti-vaccine sentiment – not just online, but with demonstrations taking place around the world.46
Transparency and openness about the vaccination process is one way of overcoming such mistrust. This is partly why the extremely busy team of scientists has given the BBC access to their labs over the past year.47
Finally, on 21 November, the independent data safety committee was ready to reveal the Oxford-AstraZeneca findings. But the results were surprising – and more complex than expected. Whereas Pfizer and Moderna had one efficacy figure from one big trial each, the Oxford jab ended up with three numbers: 70% overall, with the two full doses giving 62% protection while the smaller group, who were given that initial half dose had the highest protection, had 90%. It was a result no one had expected.48
Crucially, no one who got the vaccine was hospitalised or got seriously ill due to Covid. Whereas in the control group there were 10 serious cases and one death. The fact that those who had unwittingly been given the initial half dose from Italy showed stronger protection is intriguing. It may be caused by the immune system being primed more gradually, but the scientists can’t yet explain it. Also, all the volunteers in this group were under 55.49
Real trouble started with this death that was argued to be as a result of blood clotting linked to the vaccine. Oxford/AstraZeneca refused to accept this possible linkage. According to Ewen Callaway, the analysis of the result produced both interesting and head-scratching effects. “A highly anticipated COVID-19 vaccine has delivered some encouraging — but head-scratching — results. The vaccine developed by the University of Oxford, UK, and pharmaceutical giant AstraZeneca was found to be, on average, 70% effective in a preliminary analysis of phase III trial data, the developers announced in a press release on 23 November [2020].”50 But the analysis found a striking difference in efficacy depending on the amount of vaccine delivered to a participant. A regimen consisting of 2 full doses given a month apart seemed to be just 62% effective. But, surprisingly, participants who received a lower amount of the vaccine in the first dose and then the full amount in the second dose were 90% less likely to develop COVID-19 than were participants in the placebo arm.51
It was this discepancy in the result produced that was at the heart of the controversy. The argument is whether those who died from blood clot was as a result of (first) lower dosage or the second dosage or vice versa.
The Oxford–AstraZeneca vaccine is made from a cold-causing adenovirus that was isolated from the stool of chimpanzees and modified so that it no longer replicates in cells. When injected, the vaccine instructs human cells to produce the SARS-CoV-2 spike protein — the immune system’s main target in coronaviruses. The vaccine entered phase III efficacy trials before other front runners, including Pfizer and Moderna, and trials are continuing in countries including the United States, South Africa, Japan and Russia. The 23 November analysis is based on 131 COVID-19 cases among more than 11,000 trial participants in the United Kingdom and Brazil, up to 4 November.52
Overall, the developers found that the 2-dose vaccine had an efficacy of 70%, when measured 2 weeks after participants received their second dose. But that figure is an average of the 62% and 90% efficacy from the two dosing regimens. “90% is pretty good, but the 62% for the second tested regimen are not that impressive,” said Florian Krammer, a virologist at Icahn School of Medicine at Mount Sinai in New York City, on Twitter.53
A top priority for researchers is understanding why the vaccine seems to have performed so much better with a lower first dose. One explanation could lie in the data: the trial might not have been big enough to gauge the differences between the two regimens, in which case the differences might vanish once more cases of COVID-19 are detected, says Luk Vandenberghe, a virologist at the Massachusetts Eye and Ear institute and Harvard Medical School in Boston. The more effective ‘half-dose, full dose’ results were based on 2,741 trial participants, whereas the less efficacious arm included 8,895 volunteers. The press release did not specify in which group cases occurred.54
But, if the differences are real, researchers are eager to understand why. “I don’t think it’s an anomaly,” says Katie Ewer, an immunologist at Oxford’s Jenner Institute who is working on the vaccine. “I’m keen to get into the lab and start thinking about how we address that question.” She has two leading theories for why a lower first dose might have led to better protection against COVID-19. It’s possible that lower doses of vaccine do a better job at stimulating the subset of immune cells called T cells that support the production of antibodies, she says.55
Another potential explanation is the immune system’s response to the chimpanzee virus. The vaccine triggers a reaction not only to the SARS-CoV-2 spike protein, but also to components of the viral vector. It’s possible that the full first dose blunted this reaction, says Ewer. She plans to look at antibody responses to the chimpanzee virus to help address this question.56
“This is a plausible explanation,” says James Wilson, a virologist at the University of Pennsylvania in Philadelphia who pioneered the use of adenoviruses for vaccines in the 1990s. By giving a half-dose first, “it is possible that AstraZeneca threaded the needle with their dosing”, he adds.57
AstraZeneca has said a review of safety data of 17 million people vaccinated with its Covid-19 vaccine has shown no evidence of an increased risk of blood clots. “A careful review of all available safety data of more than 17 million people vaccinated in the European Union and UK with Covid-19 Vaccine AstraZeneca has shown no evidence of an increased risk of pulmonary embolism, deep vein thrombosis or thrombocytopenia, in any defined age group, gender, batch or in any particular country,” the company said. The drugmaker said there have been only 37 reports of blood clots among the more than 17 million people who have received the vaccine across the EU and Britain, which may have been just a coincidence.58
The big question is whether there is coincidence in science, something which philosophy of science has not been able to answer till date.
In a University of Oxford website the argument was made that “It is recognised that a vaccine is urgently needed to prevent people from becoming severely ill and dying from COVID-19. The aim of the UK COVID-19 vaccination programme is to protect those who are most at risk. In response to the COVID-19 pandemic, scientists around the world have come together to focus their efforts on developing vaccines to prevent people from becoming infected with the coronavirus. Over 270 vaccines are in various stages of development, and some of these utilise similar technologies to existing vaccines in use, whilst others involve newer approaches.59
Although clinical trials have been completed more rapidly during the pandemic, this has been achieved by overlapping the different stages (phase 1, 2 and 3) of clinical testing rather than completing them sequentially. Assessment of safety has not been compromised and the trials have been subject to the same strict regulatory requirements as any other vaccine studies. Each of the vaccines that has received or is under review for temporary licensing have been tested in trials with over 20,000 people, collecting many months of safety follow-up data. In many cases, these trials are larger than trials for other drugs and vaccines which have been licensed.”60
In the UK, two vaccines are currently in use, following regulatory approval. These are the BNT162b2 vaccine, developed by Pfizer and BioNTech and the ChAdOx1 nCoV-19 (AZD1222), developed by the University of Oxford and AstraZeneca. Both of these vaccines have been authorised for emergency use by the Medicines and Healthcare products Regulatory Agency (MHRA), as well as a vaccine developed by Moderna, which is expected to be available from Spring 2021.61
The ChAdOx1 nCoV-19 vaccine was developed by the University of Oxford and AstraZeneca. The vaccine works by delivering the genetic code of the SARS-CoV-2 spike protein to the body’s cells, similarly to the BNT162b2 vaccine. Once inside the body, the spike protein is produced, causing the immune system to recognise it and initiate an immune response. This means that if the body later encounters the spike protein of the coronavirus, the immune system will recognise it and destroy it before causing infection.62
This Oxford-AstraZeneca vaccine uses the ChAdOx1 technology, which has been developed and optimised by the Jenner Institute over the last 10 years. This type of vaccine technology has been tested for many other diseases such as influenza (flu) and Middle East Respiratory Syndrome (MERS), another type of coronavirus.63
The ChAdOx1 nCoV-19 vaccine has been tested by the University of Oxford in clinical trials of over 23,000 people in the UK, Brazil and South Africa. AstraZeneca are also running a further trial with 40,000 people in the USA, Argentina, Chile, Columbia and Peru.64
Interim results from the UK and Brazil trials showed that the vaccine can prevent 70.4% of COVID-19 cases. This was calculated across two different groups of people, who received two different dose regimens. The vaccine was shown to prevent 73% of cases in individuals with at least one underlying health condition. The vaccine has also been shown to produce similar immune responses in older adults when compared with young, healthy individuals, although the efficacy data for this group is not yet available. The ChAdOx1 nCoV-19 vaccine is given as a two-dose course, which is given as an injection into the upper arm. The second dose is given 4-12 weeks after the first dose.65
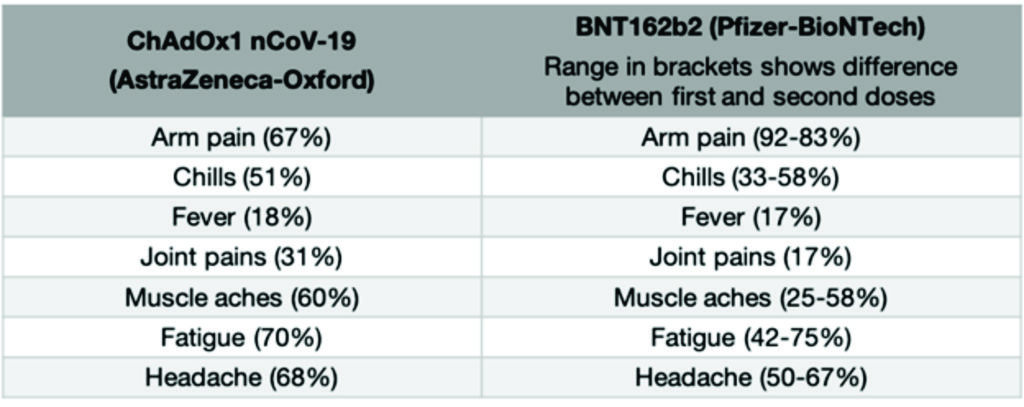
Because vaccines work by triggering your immune system to produce a reaction, you can however have side effects after you receive the vaccine that feel similar to having a real infection. Things like having a fever, or feeling achey, or getting a headache (often described as “flu-like” symptoms) are common after receiving many vaccines and this is the same for the approved COVID-19 vaccines. Having these symptoms means that your immune system is working as it should be. Usually, these symptoms last a much shorter time than a real infection would (most are gone within the first 1-2 days). The common side effects associated with the currently approved vaccines are below. These symptoms generally last 1-2 days following vaccination.
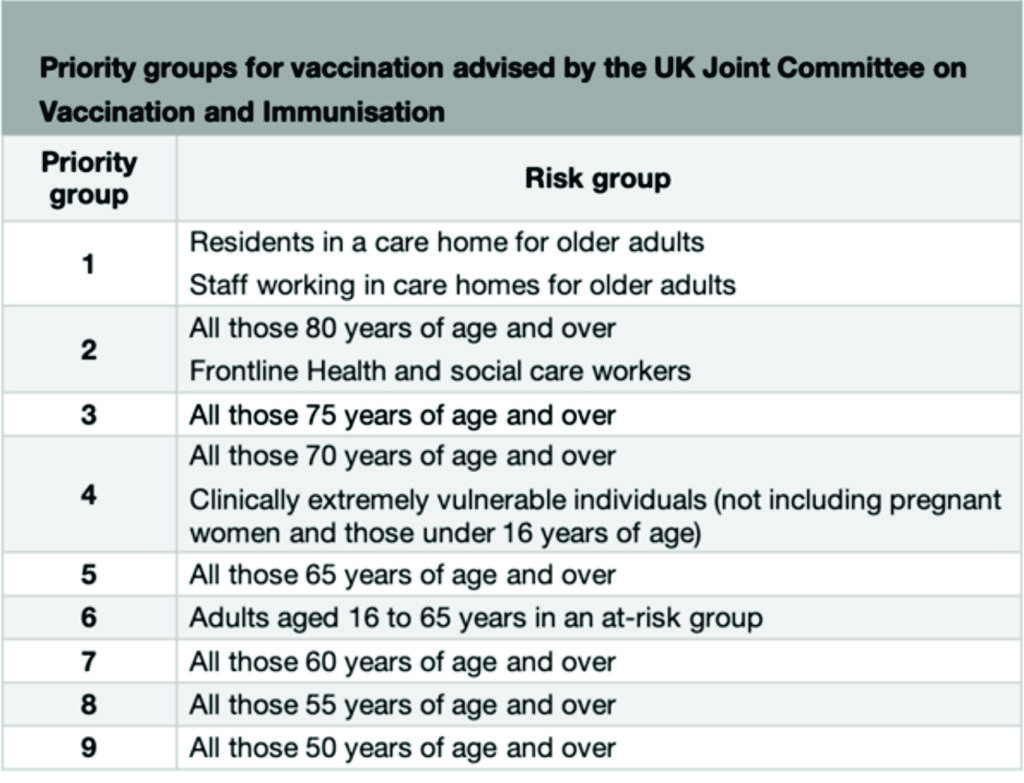
Following advice from the UK Joint Committee on Vaccination and Immunisation, the programme will vaccinate people in the order of highest risk of severe COVID-19 disease, due to occupation, age or other risk factors. The categories are outlined below:
At-risk groups included in priority group 6 are:
- Chronic respiratory disease (including severe asthma)
- Chronic heart disease and vascular disease
- Chronic kidney disease
- Chronic liver disease
- Chronic neurological disease
- Diabetes
- Immunosuppression
- Dysfunction of the spleen
- Morbid obesity
- Severe mental illness
- Adult carers
- Younger adults in long-stay care homes
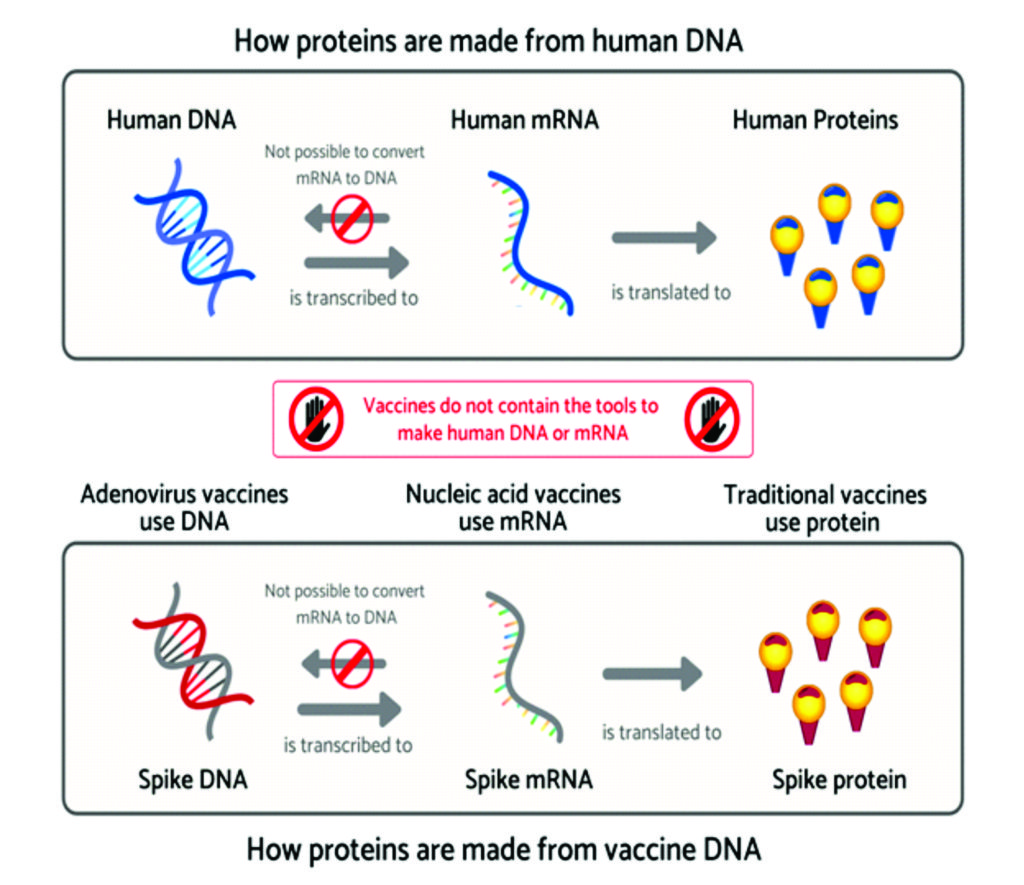
Newer vaccines such as mRNA vaccines and viral vectored vaccines, including the Oxford ChAdOx1 nCoV-19 vaccine differ from many traditional vaccines in the way they activate the immune system. Most traditional vaccines inject the antigen (part of the disease that stimulates an immune response) directly into the body. In contrast, these two newer approaches deliver the genetic instructions for the antigen to the body’s cells. The cells then manufacture the antigen which goes on to stimulate the immune response. Injecting genetic material has raised questions about the use of these vaccines, such as whether they can modify the DNA of those receiving them. Here, we will explain why this is not possible.66
A Reuters group of reporters also provided a special report that offers insights into what might have happened within the University of Oxford team of scientists working on the controversial vaccine.
The reporters reported that “On June 5 [2020], researchers at the University of Oxford quietly made a change to a late-stage clinical trial of their COVID-19 vaccine. In an amendment noted in a document marked CONFIDENTIAL, they said they were adding a new group of participants.67
The adjustment might seem minor in a large-scale study. But it masked a mistake that would have potentially far-reaching consequences: Many of the United Kingdom trial subjects had inadvertently been given only about a half dose of the vaccine.68 The new volunteers would now receive the correct dose. The trial continued.69
Much was riding on the Oxford vaccine, a British-led endeavour also involving UK drugs firm AstraZeneca. Prime Minister Boris Johnson’s government was desperate for a success story after its early mishandling of the pandemic contributed to one of the world’s highest death tolls from COVID-19 – around 65,000 by mid-December. The government has secured 100 million doses.70
On Nov[ember]. 23, [2020] Oxford and AstraZeneca delivered positive news. They announced that the regimen of a half dose followed by a full dose booster appeared to be 90% effective in preventing COVID-19. Two full doses scored 62%. Oxford researchers have said they aren’t certain why the half-dose regimen was much more effective.71
Some experts say the dosing discrepancy raises doubts about the robustness of the study’s findings. And they worry about another acknowledged peculiarity of the study: The half-dose regimen wasn’t tested on anyone over 55 – the group considered at high risk from COVID-19. In contrast, a vaccine produced by Pfizer/BioNTech was tested on thousands of people over 65, with an efficacy of 94%.72
John Moore, a professor of microbiology and immunology at Weill Cornell Medical College in New York, said there needed to be a better understanding of how the Oxford trial unfolded. “When you get corporate and academic scientists saying different things, it doesn’t give you the impression of confidence in what they’re doing,” he told Reuters. “Was the dosing thing a mistake or not?”73
Now a Reuters review of hundreds of pages of clinical trial records, as well as interviews with scientists and industry figures, provides the most detailed account to date of what went wrong with the dosing in the Oxford/AstraZeneca vaccine study. The review found that Oxford researchers were responsible for what their own clinical trial documents called “a potency miscalculation.”74
For Oxford and AstraZeneca, the stakes could not be higher. They hope to produce up to three billion doses of the low-cost vaccine by the end of next year, enough to inoculate much of the world, including many of its poorest inhabitants. For months, scientists at Oxford have been busily promoting the experimental vaccine’s prospects in bullish terms – beginning even before the first human test subjects were injected with the experimental vaccine.75
In an interview that appeared on April 11 in Britain’s The Times newspaper, Sarah Gilbert, one of the vaccine’s chief researchers at Oxford, said she was 80% certain her team would be able to produce a successful vaccine, possibly as early as September. That was 12 days before a clinical trial to test its safety began.76
Oxford didn’t answer detailed questions for this story, but provided a statement saying the trials have been “conducted under the strict national, ethical and regulatory requirements.” It added that “all trial protocols and trial amendments have been subject to review and approval by the relevant authorities. All safety data have been reviewed regularly” by regulators.77
A spokesman for AstraZeneca referred questions about the UK clinical trial to Oxford, which sponsored it. A spokeswoman for Britain’s regulator, the Medicines and Healthcare products Regulatory Agency (MHRA), declined to answer questions about the Oxford/AstraZeneca dosing issue. “Our rolling review is ongoing,” she said, “so this information is currently commercially confidential.”78 The UK’s Department of Health and Social Care declined to comment.79
On Dec. 8, Oxford published an interim analysis of its trial results and more than 1,100 pages of supplementary documents in the scientific journal The Lancet. These show that measuring the concentration of viral materials can be tricky, and they shed light on the chain of events leading up to the dosing discrepancy.80
According to the Oxford documents, in May researchers received a vaccine delivery from Italy’s IRBM/Advent — one of the contract manufacturers Oxford enlisted to complement its own vaccine production. The late-stage trial of the Oxford vaccine was about to begin.81
The shipment, batch K.0011, had undergone the Italian company’s quality check using an established genetic test – quantitative PCR, or qPCR – to determine viral matter per milliliter.82
Oxford ran its own analysis for good measure. The university had been using a different method known as spectrophotometry, which measures viral material in liquids based on how much ultraviolet light the viral matter absorbs.83
Oxford’s measurement showed that the batch was more potent than the Italian manufacturer had found, the documents show. Oxford trusted its own result and wanted to remain consistent with a measuring tool it had used throughout an earlier trial phase. So it asked Britain’s drugs regulator for permission to reduce the volume of vaccine injected into trial participants from the K.0011 batch. Permission was granted.84
“The decisions about dosing were all done in discussion with the regulator. So when we started the trial, we had some discrepancies in the measurement of the concentration of virus in the vaccine,” Andrew Pollard, the Oxford trial’s chief investigator, told Reuters.85
A spokeswoman for the regulator declined to discuss when it first became aware of the dosing issue.86
Trial participants who received shots from the Italian batch displayed milder than normal expected side effects, such as fever and fatigue. AstraZeneca executive vice president Mene Pangalos said the dose was measured incorrectly. “It ended up being half the dose,” he told Reuters. He called the mistake “serendipity,” given that data analysis later indicated the half dose, followed by a full-dose booster shot, was much more effective than two full doses.87 He also recently told the BBC: “There is no doubt I think that we would have run the study a little bit differently if we had been doing it from scratch. But ultimately, it is what it is.”88
IRBM/Advent told Reuters there was no manufacturing issue with the batch. The company said in a written statement that the measuring mishap was “the result of a change in the testing method” used to confirm the potency of the dose “once the material had been shipped.”89
The documents published in The Lancet confirm that the error lay with the Oxford researchers. A common emulsifier, polysorbate 80, used in vaccines to facilitate mixing, had interfered with the ultraviolet-light meter that measures the quantity of viral material, according to the documents. As a result, the vaccine’s viral concentration was overstated and Oxford ended up administering half doses of vaccine, believing they were full doses.90
The documents don’t give a detailed timeline, nor do they show that Oxford informed AstraZeneca at the time. But they do show that Oxford contacted the British regulator again, this time seeking approval to change its measuring method to the one used by the Italians, and to figure out how to proceed with a late-stage trial that had begun with participants receiving the wrong dose. The documents don’t provide full details of the communication between Oxford and the regulator.91
However, there was a startling revelation in the report by the Reuters reporters
Deep within the more than 1,100 pages of supplemental appendices published in The Lancet appeared a description of the dosing discrepancy — “a potency miscalculation.” That admission is contained in a “Statistical Analysis Plan” by Oxford and AstraZeneca dated Nov. 17.92 Six days later, Oxford and AstraZeneca announced the interim results of their clinical trials in the UK and in Brazil. “Oxford University breakthrough on global COVID-19 vaccine,” was the headline of an Oxford press release.93
AstraZeneca’s news release was more muted. “Two different dosing regimens demonstrated efficacy with one showing a better profile,” it stated. (Ibid) In interviews about the results with Reuters and the New York Times, AstraZeneca’s Pangalos spoke of “serendipity,” a “useful mistake” and a “dosing error.”94 But the firm’s chief executive officer, Pascal Soriot, told Bloomberg: “People call it a mistake — it was not a mistake.” A spokesman for AstraZeneca declined to comment on the statements.95
Meanwhile, the two scientists leading Oxford’s development of the vaccine — Sarah Gilbert and Adrian Hill — suggested that the half-dose was not administered by mistake. They didn’t provide evidence. Gilbert, an Oxford vaccinology professor, said it was normal for researchers to look at different dose levels during vaccine trials. “It wasn’t a mix-up in dosing,” she told the Financial Times in an article published on Nov. 27.96 A few days later, Hill told Reuters it was a conscious decision by researchers to administer a lower dose. “There had been some confusion suggesting that we didn’t know we were giving a half dose when we gave it — that is really not true,” he said.97
Gilbert and Hill together have about a 10% stake in a private biotech firm called Vaccitech that was spun out of Oxford University, according to a filing with Companies House, the UK’s companies’ registry, dated Oct. 29. According to a spokeswoman for Vaccitech, the company transferred its rights to the vaccine to Oxford University’s research commercialization arm in exchange for a share of the revenue. “If the vaccine is successful then all shareholders and investors in the company could potentially indirectly benefit,” she wrote in an email.98 Hill and Gilbert didn’t respond to detailed questions for this article.99
Some big questions are these: Could there have been a cover-up about the dosing discrepancy among the scientists? Or was just a pure mistake or error? Why are the Oxford scientists unwilling to even entertain the idea of a mistake or error in their work? Why not go all over again in reviewing their works to see whether there may have been a point (currently overlooked) where this mistake or error could have happened? Was this unwillingness to review their works all over again not against openess and transparency which the group has hitherto demonstrated? Are there plausible deniability for such mistakes or errors? Were there conflicts of interests as reported in the Reuters’ special report above? Are these conflicts of interests jurisprudentially criminal in nature? Can they be prosecuted?
What has become apparent from the above analysis is that there is no hiding place once a slip, mistake or error has been made either deliberately or inadvertently. The only option is to come out clean and openly rather than hide behind stubborness or obstinacy that further escalates the crisis. It is obvious that the Oxford team of scientists were trying to hide their mistake or error from public scrutiny. Several officials contacted by the Reuters’ reporters declined to comment or answer questions put to them which can be interpreted to mean they are aware of something having gone wrong but playing safe. They were probably clumsy at doing this given the inquisitiveness through the media: print and social media some of which rely on sensational reporting to attract wider followership. This is another whole dimension to the controversy: how media fed into the crisis and escalated it to the stratosphere. It might not be out of place to suggest that the EU countries that have rejected the vaccine acted while hiding behind the preponderant weight of public opinion aggregated through the media in order to quickly divert public opinion from their hitherto poor performance in handling the coronavirus pandemic when is was at its raging peak.
The Political Tango between Britain and EU
The political angle to the controversy was thrown open by none other than an article that appeared in prestigious and respected The Lancet medical journal in February 2021 by Rob Hyde.
EU Commission President Ursula von der Leyen made a startling speech last week in relation to widespread criticism of how Europe has so far handled the COVID1-19 vaccine roll-out. “We were late in granting authorisation. We were too optimistic about mass production. And maybe we also took for granted that the doses ordered would actually arrive on time”, she said. Views differ widely, however, on whether the EU did anything wrong in how it went about securing vaccines for its citizens.100
The EU aims to both secure vaccine supplies, and also provide support for the development of further vaccines in the union. Its approach is centralised. The Commission acts on behalf of all 27 member states by negotiating advance purchase agreements with vaccine manufacturers. Approval is then made by the European Medical Agency (EMA).101
The benefit of this approach is that, in theory, the EU can secure lower prices. As a huge bloc, the EU should have significant bargaining power in negotiations. Another advantage is that vaccines are ordered on behalf of all EU countries. This stops wealthier states such as Germany, France, and the Netherlands buying up all available vaccines, leaving nothing for economically weaker states such as Romania and Bulgaria.102
According to the EU’s website, its vaccine strategy is funded via “a significant part” of the €2·7 billion emergency support instrument. This money is used as a down payment on the vaccines that EU member states will then purchase. Other funds are also being used to boost production capacity. The aim is to make millions, or even billions, of doses of a vaccine.103
It normally takes around 10 years to develop a vaccine. The Commission’s strategy, however, is an accelerated programme that is designed to develop the vaccine and make it available within 12–18 months.104
But just how accelerated is this programme? So far, the EU has concluded contracts for COVID-19 vaccines with Moderna (160 million doses), Sanofi-GSK (300 million doses), AstraZeneca (400 million doses), Johnson & Johnson (400 million doses), CureVac (405 million doses), and Pfizer–BioNTech (600 million doses). It has also had exploratory talks with the pharmaceutical companies Novavax and Valneva. However, the EMA has so far granted conditional market authorisation only to the AstraZeneca, Moderna, and Pfizer–BioNTech vaccines.105
Further questions have been asked about the speed at which people are being vaccinated, particularly in comparison with the UK. The UK was the first country to approve the Pfizer–BioNTech COVID-19 vaccine, on Dec 2, 2020, and later rolled out the AstraZeneca–Oxford and Moderna vaccines. The EU, however, did not approve the Pfizer-BioNTech vaccine until Dec 20. As of Feb 15, more than 15 million people in the UK had received at least one dose of the vaccine; around 23% of the population. In the EU, however, only 4·4 people per 100 000 have.106
A key explanation for the gap between the EU and UK vaccine roll-outs is down to the British–Swedish company, AstraZeneca, and the controversial vaccine procurement contacts. In summary, the UK got there first, placing an order in May, 2020, for 100 million doses. It also made sure the contracts had clear delivery dates.107
The EU signed a deal for 300 million doses of the vaccine, but not until August, 2020. Furthermore, according to reports in the German newspaper Bild, procurement expert Gerd Kerkhoff concluded the contract was “a complete disaster”. The contract had 18 “best reasonable efforts clauses”, which legally allowed the company not to deliver on agreed dates, if it said it was not able to.108
On Jan 29, 2021, the EU began trying to gain greater control over access to the vaccine. It announced that EU-based manufacturing plants, such as the Pfizer production plant in Puurs, Belgium, would now have to request authorisation to be able to export the vaccine. The EU even threatened to stop the flow of vaccine from Ireland to Northern Ireland, although this proposal was swiftly withdrawn after provoking outrage not just in London and Belfast, but also in Dublin. European media quickly slammed the Commission for threatening the Northern Ireland peace process.109
Fierce criticism of von der Leyen has also come from the usually pro-EU media in Germany—the very country which von der Leyen once served as defence minister. Die Zeit newspaper’s Alan Posener said that “if the British were still EU citizens, they would be like us: instead of having vaccinations, simply waiting for Godot”. Der Spiegel said that the EU had attempted to secure the vaccines in a “hare-brained manner, as if it were a summer sale, a bargain hunt on a whim.” Peter Tiede of the daily Bild newspaper claimed that von der Leyen had “disgraced Europe”.110
Not everyone, however, shares these views. Ellen ‘t Hoen, is a lawyer and public health advocate at research group Medicines, Laws and Policy, and is former policy director for Médecins sans Frontières’ Campaign for Access to Essential Medicines. Speaking to The Lancet, she said people should be cautious before envying the UK’s approach. “What is the UK going to gain if other countries in Europe don’t have the vaccine? It only works if you say ‘everyone for themselves’ and you build a big wall around yourself.”111
It is evident that the Oxford/AstraZeneca vaccine faced two major problems which come to constitute a hydra-headed monster or to be specific a Janus-faced monster: the logistic problem associated with the demand-and-supply disequilibria and the two-dose efficacy disequilibria/discrepancy. Oxford/AstraZeneca has not been able to resolve any of the problem satisfactorily. However, the saving grace so far has been that the vaccine is already on the global market shelf and is being administered in several countries outside those countries that rejected it, Through this “escape route” Oxford/AstraZeneca can recoup its losses but the damage to its reputation would be long lasting.
There were two further interesting pieces of research works published in The Lancet during the controversy which go to shed more light on the vaccine especially as regard the trials carried out in the United Kingdom, Brazil and South Africa about the efficacy of the vaccine on different age groups.
In early January 2021, The Lancet medical journal published an article by a group of scientists. The research was funded by UK Research and Innovation, National Institutes for Health Research (NIHR), Coalition for Epidemic Preparedness Innovations, Bill & Melinda Gates Foundation, Lemann Foundation, Rede D’Or, Brava and Telles Foundation, NIHR Oxford Biomedical Research Centre, Thames Valley and South Midland’s NIHR Clinical Research Network, and AstraZeneca.
The aim of the report is finding “A safe and efficacious vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), if deployed with high coverage, could contribute to the control of the COVID-19 pandemic.”112
The primary objective was to evaluate the efficacy of ChAdOx1 nCoV-19 vaccine against NAAT-confirmed COVID-19. The primary outcome was virologically confirmed, symptomatic COVID-19, defined as a NAAT-positive swab combined with at least one qualifying symptom (fever ≥37·8°C, cough, shortness of breath, or anosmia or ageusia).113
The report evaluated “the safety and efficacy of the ChAdOx1 nCoV-19 vaccine in a pooled interim analysis of four trials.”114
Findings show that: “Between April 23 and Nov 4, 2020, 23 848 participants were enrolled and 11 636 participants (7548 in the UK, 4088 in Brazil) were included in the interim primary efficacy analysis. In participants who received two standard doses, vaccine efficacy was 62·1% (95% CI 41·0–75·7; 27 [0·6%] of 4440 in the ChAdOx1 nCoV-19 group vs71 [1·6%] of 4455 in the control group) and in participants who received a low dose followed by a standard dose, efficacy was 90·0% (67·4–97·0; three [0·2%] of 1367 vs 30 [2·2%] of 1374; pinteraction=0·010). Overall vaccine efficacy across both groups was 70·4% (95·8% CI 54·8–80·6; 30 [0·5%] of 5807 vs 101 [1·7%] of 5829). From 21 days after the first dose, there were ten cases hospitalised for COVID-19, all in the control arm; two were classified as severe COVID-19, including one death. There were 74 341 person-months of safety follow-up (median 3·4 months, IQR 1·3–4·8): 175 severe adverse events occurred in 168 participants, 84 events in the ChAdOx1 nCoV-19 group and 91 in the control group. Three events were classified as possibly related to a vaccine: one in the ChAdOx1 nCoV-19 group, one in the control group, and one in a participant who remains masked to group allocation.”115
In participants who received two standard doses, efficacy against primary symptomatic COVID-19 was consistent in both the UK (60·3% efficacy) and Brazil (64·2% efficacy), indicating these results are generalisable across two diverse settings with different timings for the booster dose (with most participants in the UK receiving the booster dose more than 12 weeks after the first dose and most participants in Brazil receiving their second dose within 6 weeks of the first). Exploratory subgroup analyses included at the request of reviewers and editors also showed no significant difference in efficacy estimates when comparing those with a short time window between doses (<6 weeks) and those with longer (≥6 weeks), although further detailed exploration of the timing of doses might be warranted.116
Efficacy of 90·0% seen in those who received a low dose as prime in the UK was intriguingly high compared with the other findings in the study. Although there is a possibility that chance might play a part in such divergent results, a similar contrast in efficacy between the LD/SD and SD/SD recipients with asymptomatic infections provides support for the observation (58·9% [95% CI 1·0 to 82·9] vs 3·8% [−72·4 to 46·3]). Exploratory subgroup analyses, included at the request of reviewers and editors, that were restricted to participants aged 18–55 years, or aligned (>8 weeks) intervals between doses, showed similar findings. Use of a low dose for priming could provide substantially more vaccine for distribution at a time of constrained supply, and these data imply that this would not compromise protection. While a vaccine that could prevent COVID-19 would have a substantial public health benefit, prevention of asymptomatic infection could reduce viral transmission and protect those with underlying health conditions who do not respond to vaccination, those who cannot be vaccinated for health reasons, and those who will not or cannot access a vaccine, providing wider benefit for society. However, the wide CIs around our estimates show that further data are needed to confirm these preliminary findings, which will be done in future analyses of the data accruing in these ongoing trials.117
In our studies, the demographic characteristics of those enrolled varied between countries. In the UK, the enrolled population was predominantly white and, in younger age groups, included more female participants due to the focus on enrolment of health-care workers. This is a typically lower risk population for severe COVID-19. The demographic profile combined with the weekly self-swabbing for asymptomatic infection in the UK results in a milder case-severity profile. In Brazil, there was a larger proportion of non-white ethnicities, and again the majority of those enrolled were health-care workers.118
We have previously reported on the local and systemic reactogenicity of ChAdOx1 nCoV-19 and shown that it is tolerated and that the side-effects are less both in intensity and number in older adults, with lower doses, and after the second dose. Although there were many serious adverse events reported in the study in view of the size and health status of the population included, there was no pattern of these events that provided a safety signal in the study. Three cases of transverse myelitis were initially reported as suspected unexpected serious adverse reactions, with two in the ChAdOx1 nCoV-19 vaccine study arm, triggering a study pause for careful review in each case. Independent clinical review of these cases has indicated that one in the experimental group and one in the control group are unlikely to be related to study interventions, but a relationship remained possible in the third case. Careful monitoring of safety, including neurological events, continues in the trials. All safety data will be provided to regulators for review.119
In this interim analysis, we have not been able to assess duration of protection, since the first trials were initiated in April, 2020, such that all disease episodes have accrued within 6 months of the first dose being administered. Further evidence will be required to determine duration of protection and the need for additional booster doses of vaccine.120
The results presented in this Article constitute the key findings from the first interim analysis, which are provided for rapid review by the public and policy makers. In future analyses with additional data included as they accrue, we will investigate differences in key subgroups such as older cohorts, ethnicity, dose regimen, and timing of booster vaccines, and we will search for correlates of protection.121
Until widespread immunity halts the spread of SARS-CoV-2, physical distancing measures and novel therapies are needed to control COVID-19. In the meantime, an efficacious vaccine has the potential to have a major impact on the pandemic if used in populations at risk of severe disease. Here, we have shown for the first time that a viral vector vaccine, ChAdOx1 nCoV-19, is efficacious and could contribute to control of the disease in this pandemic.122
In early March 2021, The Lancet medical journal published another article by the same group of scientists already mentioned above. The report is an independent research funded by NIHR, UK Research and Innovation, the Bill & Melinda Gates Foundation, the Lemann Foundation, Rede D’Or, the Brava and Telles Foundation, and the South African Medical Research Council.123
Their research findings reveal that “Between April 23 and Dec 6, 2020, 24 422 participants were recruited and vaccinated across the four studies, of whom 17 178 were included in the primary analysis (8597 receiving ChAdOx1 nCoV-19 and 8581 receiving control vaccine). The data cutoff for these analyses was Dec 7, 2020. 332 NAAT-positive infections met the primary endpoint of symptomatic infection more than 14 days after the second dose. Overall vaccine efficacy more than 14 days after the second dose was 66·7% (95% CI 57·4–74·0), with 84 (1·0%) cases in the 8597 participants in the ChAdOx1 nCoV-19 group and 248 (2·9%) in the 8581 participants in the control group. There were no hospital admissions for COVID-19 in the ChAdOx1 nCoV-19 group after the initial 21-day exclusion period, and 15 in the control group. 108 (0·9%) of 12 282 participants in the ChAdOx1 nCoV-19 group and 127 (1·1%) of 11 962 participants in the control group had serious adverse events. There were seven deaths considered unrelated to vaccination (two in the ChAdOx1 nCov-19 group and five in the control group), including one COVID-19-related death in one participant in the control group. Exploratory analyses showed that vaccine efficacy after a single standard dose of vaccine from day 22 to day 90 after vaccination was 76·0% (59·3–85·9). Our modelling analysis indicated that protection did not wane during this initial 3-month period. Similarly, antibody levels were maintained during this period with minimal waning by day 90 (geometric mean ratio [GMR] 0·66 [95% CI 0·59–0·74]). In the participants who received two standard doses, after the second dose, efficacy was higher in those with a longer prime-boost interval (vaccine efficacy 81·3% [95% CI 60·3–91·2] at ≥12 weeks) than in those with a short interval (vaccine efficacy 55·1% [33·0–69·9] at <6 weeks). These observations are supported by immunogenicity data that showed binding antibody responses more than two-fold higher after an interval of 12 or more weeks compared with an interval of less than 6 weeks in those who were aged 18–55 years (GMR 2·32 [2·01–2·68]).”124
The analysis presented here provides strong evidence for the efficacy of two standard doses of the vaccine, which is the regimen approved by the MHRA and other regulators. Following regulatory approval, a key question for policy makers to plan the optimal approach for roll-out is the optimal dose interval, which is assessed in this report through post-hoc exploratory analyses. Two criteria that contribute to decision making in this area are the effect of prime-boost interval on protection after the second dose and the degree to which the vaccinated individual is at risk of infection during the time period before the booster dose, if there were either reduced efficacy with a single dose or rapid waning of efficacy before the second vaccination.125
Exploratory analyses are presented in this report that show protection with dosing intervals from less than 6 weeks to 12 weeks or more and that a longer interval provides better protection after a booster dose without compromising protection in a 3-month period before the second dose is administered.126
In exploratory analyses, a single standard dose of ChAdOx1 nCoV-19 had an efficacy of 76·0% (95% CI 59·3 to 85·9) against symptomatic COVD-19 in the first 90 days after vaccination, with no significant waning of protection during this period. It is not clear how long protection might last with a single dose because follow-up is limited to the time periods described here, and, for this reason, a second dose of vaccine is recommended.127
A second dose of ChAdOx1 nCoV-19 induces increased neutralising antibody levels and is probably necessary for long-lasting protection. However, where there is low supply of vaccine, a policy of initially vaccinating a larger cohort with a single dose might provide better overall population protection than vaccinating half the number of individuals with two doses in the short term. With the evidence available at this time, it is anticipated that a second dose is still required to potentiate long-lived immunity. Recent modelling of delayed boosting suggests that even in the presence of substantial waning of first-dose efficacy, programmes that delay a second dose to vaccinate a larger proportion of the population result in greater immediate overall population protection.128
In our study, vaccine efficacy after the second dose was higher in those with a longer prime-boost interval, reaching 81·3% in those with a dosing interval of 12 weeks or more versus 55·1% in those with an interval of less than 6 weeks. Point estimates of efficacy were lower with shorter dosing intervals, although it should be noted that there is some uncertainty because the CIs overlap. Higher binding and neutralising antibody titres were observed in sera with the longer prime-boost interval, suggesting that, assuming there is a relationship between the humoral immune response and efficacy, these might be true findings and not artifacts of the data. Greater protective efficacy associated with stronger immune responses after a wider prime-boost interval have been seen with other vaccines such as those for influenza, Ebola virus disease, and malaria.129
The findings presented here for the ChAdOx1 nCoV-19 vaccine are consistent with policy recommendations in different countries to use dose intervals of 4–12 weeks for this vaccine.130
Finally the authors declared that “It is not clear what effect each of these individual sources of variation in the data have on vaccine efficacy estimates. However, the same trend seen with efficacy is also seen in the immunological data, suggesting an underlying biological mechanism.131
Vaccination programmes aimed at vaccinating a large proportion of the population with a single dose, with a second dose given after a 3-month period, might be an effective strategy for reducing disease, and might have advantages over a programme with a short prime-boost interval for roll-out of a pandemic vaccine when supplies are scarce in the short term. Two doses of ChAdOx1 nCoV-19 was efficacious in preventing symptomatic COVID-19. These results confirm those seen in the interim analysis of the trials.132
Despite these pieces of research work, several EU countries were not swayed nor persuaded by the findings of these researches. They still went ahead to impose the ban on the administration of the vaccine in their countries.
According to Rebecca Robbins and Benjamin Mueller, writing in New York Times, “[t]he announcement this week [November 23, 2020] that a cheap, easy-to-make coronavirus vaccine appeared to be up 90 percent effective was greeted with jubilation. “Get yourself a vaccaccino,” a British tabloid celebrated, noting that the vaccine, developed by AstraZeneca and the University of Oxford, costs less than a cup of coffee.133 But since unveiling the preliminary results, AstraZeneca has acknowledged a key mistake in the vaccine dosage received by some study participants, adding to questions about whether the vaccine’s apparently spectacular efficacy will hold up under additional testing.134
Scientists and industry experts said the error and a series of other irregularities and omissions in the way AstraZeneca initially disclosed the data have eroded their confidence in the reliability of the results. Officials in the United States have noted that the results were not clear. It was the head of the flagship federal vaccine initiative — not the company — who first disclosed that the vaccine’s most promising results did not reflect data from older people.135
The upshot, the experts said, is that the odds of regulators in the United States and elsewhere quickly authorizing the emergency use of the AstraZeneca vaccine are declining, an unexpected setback in the global campaign to corral the devastating pandemic. “I think that they have really damaged confidence in their whole development program,” said Geoffrey Porges, an analyst for the investment bank SVB Leerink.136
Michele Meixell, a spokeswoman for AstraZeneca, said the trials “were conducted to the highest standards.” In an interview on Wednesday, Menelas Pangalos, the AstraZeneca executive in charge of much of the company’s research and development, defended the company’s handling of the testing and its public disclosures. He said the error in the dosage was made by a contractor, and that, once it was discovered, regulators were immediately notified and signed off on the plan to continue testing the vaccine in different doses. Asked why AstraZeneca shared some information with Wall Street analysts and some other officials and experts but not with the public, he responded, “I think the best way of reflecting the results is in a peer-reviewed scientific journal, not in a newspaper.”137
AstraZeneca was the third company this month to report encouraging early results on a coronavirus vaccine candidate. At first glance on Monday morning, the results looked promising. Depending on the strength at which the doses were given, the vaccine appeared to be either 90 percent or 62 percent effective. The average efficacy, the developers said, was 70 percent. Almost immediately, though, there were doubts about the data.138 The regimen that appeared to be 90 percent effective was based on participants receiving a half dose of the vaccine followed a month later by a full dose; the less effective version involved a pair of full doses. AstraZeneca disclosed in its initial announcement that fewer than 2,800 participants received the smaller dosing regimen, compared with nearly 8,900 participants who received two full doses.139
The biggest questions were, why was there such a large variation in the effectiveness of the vaccine at different doses, and why did a smaller dose appear to produce much better results? AstraZeneca and Oxford researchers said they did not know. Crucial information was also missing. The company said that the early analysis was based on 131 symptomatic Covid-19 cases that had turned up in study participants. But it did not break down how many cases were found in each group of participants — those who received the half-strength initial dose, the regular-strength initial dose and the placebo. “The press release raised more questions than it answered,” said John Moore, a professor of microbiology and immunology at Weill Cornell Medical College.140
Adding to the confusion, AstraZeneca pooled the results from two differently designed clinical trials in Britain and Brazil, a break from standard practice in reporting the results of drug and vaccine trials. “I just can’t figure out where all the information is coming from and how it’s combining together,” said Natalie Dean, a biostatistician and an expert in vaccine trial design at the University of Florida. She wrote on Twitter that AstraZeneca and Oxford “get a poor grade for transparency and rigor when it comes to the vaccine trial results they have reported.”141
With AstraZeneca’s shares declining on Monday, company executives held several private conference calls with industry analysts in which they disclosed details that were not in the public announcement, including how the Covid-19 cases broke down across different groups. Such disclosures to analysts are not uncommon in the industry, but they often generate criticism about why the details were not shared with the public.142
By March 2021, the brewing controversy has gotten out of hands as several European countries including Germany, France, and Italy halted orders from AstraZeneca company. Why this three countries are important is because of their enormous weight and clout in Europe apart from the fact that they are members of the G-7 economies. This means that their individual and collective decisions can easily hurt the United Kingdom to a very large extent apart from the blow to the British prestige in European and world affairs. Spain was to later join in the abandonment of the vaccine.
The situation is binary, i.e.creating a dialectical opposite situation. The decision to stop vaccine supply from AstraZeneca is also negatively affecting the European Union itself. But the EU countries that have halted supply from the pharmaceutical giant are placing public health safety of their citizens including their national security interests at the forefront which can now be seen to have come to constitute the main rationale for kicking against the AstraZeneca vaccine even if it is just only few people that have allegedly died so far (as of the time of writing this in March 2021) from the adverse effects (such as blood clotting) of the controversial vaccine. “The European Union is facing further shortfalls in its coronavirus inoculation programme after pharmaceutical giant AstraZeneca said production problems and export restrictions would reduce planned deliveries of its vaccine.”143
The Anglo/Swedish firm’s image has already taken a hit with several countries suspending the rollout of its vaccine over blood clot fears, even as the World Health Organization said there was no reason to stop using it. It is just the latest blow for the AstraZeneca vaccine, which is the cheapest vaccine aimed at fighting back against a pandemic that has claimed more than 2.6 million lives worldwide. Germany has already reported adverse effects due to the delay, the state of Thuringia cancelling appointments and suspending a pilot project for general practitioners to administer the vaccine.144
Several countries suspended the use of AstraZeneca’s vaccine this week, with Norway reporting an “unexpected death from a brain haemorrhage” after receiving the shot. Norwegian officials added on Saturday that the country had “received several adverse event reports about younger vaccinated people with bleeding under the skin” after getting the shot. It also said it had received “three more reports of severe cases of blood clots or brain haemorrhages in younger people who have received the AstraZeneca vaccine”.145
The World Health Organization, which said its vaccines advisory committee was examining the safety data coming in, has stressed that no causal link has been established between the AstraZeneca vaccine and blood clottin. “Yes, we should continue using the AstraZeneca vaccine,” WHO spokeswoman Margaret Harris said Friday, stressing that any concerns over safety must be investigated. AstraZeneca insisted its jab was safe, adding there was “no evidence” of higher blood clot risks.146
Italy and Austria have banned the use of jabs from separate batches of AstraZeneca, and Thailand and Bulgaria said this week they would delay their rollout. Austria, the Czech Republic, Slovenia, Bulgaria and Latvia meanwhile called for EU talks to discusss “huge disparities” in vaccine distribution, according to a letter published on Saturday. “If this system were to carry on, it would continue creating and exacerbating huge disparities among member states by this summer, whereby some would be able to reach herd immunity in a few weeks while others would lag far behind,” the letter said.147
Health authorities in Denmark, Norway and Iceland on Thursday [March 11, 2021] suspended the use of AstraZeneca’s COVID-19 vaccine following reports of the formation of blood clots in some people who had been vaccinated.148 Austria earlier stopped using a batch of AstraZeneca shots while investigating a death from coagulation disorders and an illness from a pulmonary embolism.149
Denmark suspended the shots for two weeks after a 60-year-old woman, who was given an AstraZeneca shot from the same batch used in Austria, formed a blood clot and died, Danish health authorities said. Their response was also prompted by reports “of possible serious side effects” from other European countries. “It is currently not possible to conclude whether there is a link. We are acting early, it needs to be thoroughly investigated,” Health Minister Magnus Heunicke said on Twitter. The vaccine would be suspended for 14 days in Denmark. “This is a cautionary decision,” Geir Bukholm, director of infection prevention and control at the Norwegian Institute of Public Health (FHI), told a news conference.150
Iceland on Thursday suspended jabs with the vaccine as it awaited the results of an investigation by the EMA. Italy, also on Thursday, said it would suspend use of an AstraZeneca batch different to the one used in Austria.151
Some health experts said there was little evidence to suggest the AstraZeneca vaccine should not be administered and that the cases of blood clots corresponded with the rate of such cases in the general population. “The problem with spontaneous reports of suspected adverse reactions to a vaccine are the enormous difficulty of distinguishing a causal effect from a coincidence,” Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, told Reuters. Evans added that the COVID-19 disease was very strongly associated with blood clotting. Phil Bryan, head of the UK Medicines and Healthcare Products Regulatory Agency (MHRA) said reports of blood clots so far didn’t exceed what would have occurred naturally in the vaccinated population. “Available evidence does not confirm that the vaccine is the cause,” he said.152
More than 11 million doses of AstraZeneca’s vaccine have so far been administered across the UK. In a statement, AstraZeneca said it had found no evidence of an increased risk of pulmonary embolism or deep vein thrombosis in safety data of more than 10 million records, even when considering subgroups based on age, gender, production batch or country of use.153
Four other countries — Estonia, Lithuania, Luxembourg and Latvia — have stopped inoculations from the batch while investigations continue, the EMA said. The batch of 1 million doses went to 17 EU countries. Swedish authorities said they did not find sufficient evidence to stop vaccination with AstraZeneca’s jab. “There is nothing to indicate that the vaccine causes this type of blood clots,” Veronica Arthurson, head of drug safety at the Swedish Medical Products Agency, told a news conference. The Danish Medicines Agency said it had launched an investigation into the vaccine together with corresponding agencies in other EU countries and the EMA. So far, 138,148 Danes have received a shot with AstraZeneca’s vaccine in a country of 5.8 million. The Nordic country, which also uses vaccines from Pfizer-BioNTech and Moderna, is set to receive 2.6 million doses from AstraZeneca over the coming months. Denmark’s Health Authority said the final date for when it expects all Danes to have been fully vaccinated would be pushed back by four weeks to Aug. 15. Spain on Thursday said it had not registered any cases of blood clots related to AstraZeneca’s vaccine so far and would continue administering the shots.154
Despite the lack of clear and specific evidences, the dark cloud of doubt has refused to go away.
According to Eric Sagonowsky, AstraZeneca’s COVID-19 vaccine rollout has hit one pothole after another—supply shortfalls, reports of blood clots and countries suspending usage, one by one.155 And the rough ride isn’t over yet. Even as the company publicly backed its shot, citing real-world data in 17 million recipients, more countries halted their campaigns. Meanwhile, AstraZeneca was forced to dial back its delivery plans in Europe yet again, this time to 100 million doses for the first half of this year—200 million fewer than originally promised.156
In a release over the weekend, the drugmaker said it had reviewed data from around the world and found “no evidence of an increased risk of pulmonary embolism, deep vein thrombosis or thrombocytopenia, in any defined age group, gender, batch or in any particular country.”157
As of March 8, there had been 15 reports of deep vein thrombosis and 22 reports of pulmonary embolism, a number lower than would be typically expected among the 17 million people who have received the shot, the company said. “The nature of the pandemic has led to increased attention in individual cases, and we are going beyond the standard practices for safety monitoring of licensed medicines in reporting vaccine events, to ensure public safety,” AstraZeneca chief medical officer Ann Taylor said in a statement.158
Meanwhile, officials in Norway said three health workers who received the shot were being treated for bleeding, blood clots and a low platelet count, Reuters reports. Afterward, Ireland halted its use of the shot. In Italy, prosecutors seized one batch of the vaccine to investigate the death of a man after vaccination, according to the news service.On Monday, Germany suspended its use of the shot, accoding to the Associated Press. France followed a short time after.159
Plus, the company hasn’t yet solved its supply problem. Last week, AstraZeneca dramatically cut its second-quarter delivery target for Europe—to 70 million doses from 180 million doses. Back in February, a spokesperson said the company would source its global supply chain for about half of the 180 million doses scheduled to be sent to Europe during the second quarter. Now, the company says it’s aiming to deliver 100 million doses during the first half of the year, with 30 million coming in the first quarter. With the new target, AZ expects to ship 70 million doses to Europe from April to June. The company previously aimed to ship 300 million doses during the first half of the year, Reuters reports.160
In the U.S., officials have rejected requests to share its AstraZeneca vaccine doses that are stockpiled and ready for a potential FDA emergency authorization. There are fewer than 10 million doses in the stockpile, Bloomberg reports, citing sources. Before Ireland and Germany stopped vaccinations with the AZ shot, several other European countries had paused using some or all doses. Denmark paused all vaccinations, while Austria and other countries paused usage of a specific batch that had been shipped around Europe.161
On Sunday, AZ said it had seen no confirmed quality issues in any batch distributed worldwide. AstraZeneca conducts more than 60 quality tests on the product, and independent labs run another 20 studies, the company said. No tests of any batch have “shown cause for concern.” 162 The whirlwind of headlines has helped cause a perception problem for the vaccine in Europe. A recent YouGov survey found that people in Europe were less receptive to the vaccine than people in the U.K., where the shot is a point of national pride. Many AZ doses are going unused, according to data from the European Centre for Disease Prevention and Control.163
Perhaps central to the Oxford/AstraZeneca vaccine crisis was the reported failure of one of its subcontractors to fulfill its own end of the negotiated bargain. Financial Times reported that a Dutch facility in the overall contract may have been responsible for the crisis for failure to deliver a single dose to the EU bloc.
AstraZeneca’s struggle to ramp up vaccine supplies to the EU is partly because of the failure of one of the company’s key European manufacturing sites to deliver any doses to the bloc six months after the supply contract was agreed. The Dutch factory, run by subcontractor Halix, is yet to receive EU regulatory approval to supply the region even though it was named in the deal signed between AstraZeneca and the European Commission in August.164
The mystery of the Dutch factory underlines the growing questions over both AstraZeneca’s management of its EU contract and the bloc’s oversight. AstraZeneca has fallen far behind its planned vaccine deliveries to the EU, which has had a major effect on vaccination rollout. (Ibid) If it is the case that this facility is not producing for the EU then it is truly baffling. It would mean that three of the four plants listed in the original EU contract are not providing doses to the EU.165
The EU had administered 10.4 vaccine doses per 100 residents by Friday [March 12, 2021], compared with 29.7 in the US and 36.5 in the UK, according to data gathered by the Financial Times. Both the US and UK did deals with AstraZeneca earlier than the European Commission. EU officials said this week that AstraZeneca would fall roughly 10m doses short of its target to deliver 40m doses by the end of March. That goal was already well below the original supply schedule of at least 100m shots by the end of the month.166
Thierry Breton, EU industry commissioner, said on Thursday that he did not believe AstraZeneca had made “best efforts” to meet its commitments — a reference to language in the August supply contract. Concern is now growing that the British-Swedish company might also fail to deliver the 180m doses it had initially promised the EU for the second quarter of the year, half of which are due to come from outside the bloc.167
The US has so far refused to allow exports of any of the company’s US-based production, EU officials say. Supplying the EU from other countries in AstraZeneca’s worldwide production network could also be difficult.168
The Halix factory is one of two facilities — along with the Belgian plant at Seneffe — named as main sources of so-called vaccine drug substance in AstraZeneca’s contract with the commission. Pascal Soriot, the company’s chief executive, explained in an interview with European newspapers in January that the vaccine drug substance is produced in Belgium and the Netherlands and then finished and packaged into vials at plants in Germany and Italy.169
The Seneffe plant has struggled with lower-than-expected yields, while the Halix plant in the Netherlands’ Leiden Bio Science Park has produced vaccines but is still not authorised to supply them in the EU. Last week Breton visited the Halix facility — which should produce at least 5m doses a month — as part of a tour of European vaccine manufacturing sites.170
Discussions over regulatory approval for the plant from the European Medicines Agency to supply the EU market were ongoing, the officials said. Asked about the Halix situation, the commission said on Friday that the EMA was ready to fast-track authorisation of new production facilities once it received an application and the necessary information from AstraZeneca. “It is, however, the responsibility of the company to request plants to be covered by a marketing authorisation and to submit all necessary data to that effect,” it said.171 “The commission encourages the company to do so.” A spokesman for AstraZeneca said: “The approval of the site with the EMA remains on track with our original plans and we can confirm that it forms part of our delivery plans.” Halix did not respond to requests for comment.172
EU diplomats have grown increasingly agitated over how many vaccine doses Halix has actually manufactured in the meantime and what AstraZeneca is doing with the product. Officials are counting on stockpiled vaccine to be released for use in the EU once the factory is authorised. In January, the bloc introduced new discretionary controls on vaccine exports to 31 high- and middle-income countries, which Italy has already used to prevent a shipment to Australia. Brussels has clashed previously with both AstraZeneca and London over vaccine exports.173
EU officials have claimed the company has shipped vaccines produced in the EU to the UK. AstraZeneca has so far not sent doses in the other direction, even though two UK drug substance plants are referenced in the EU’s supply contract as potential sources of vaccine.174
The Halix situation also raises the question of whether the European Commission and EU member states paid sufficient attention to tracking whether the plant was on schedule to deliver. Halix did not issue a press release announcing it had been contracted to produce vaccines until December, more than three months after it was named in the AstraZeneca contract with the commission. “If it is the case that this facility is not producing for the EU then it is truly baffling,” said one EU diplomat. “It would mean that three of the four plants listed in the original EU contract are not providing doses to the EU.”175
It is obvious that Oxford/AstraZeneca has inadvertently shot itself in the foot or on the face with the evident mismanagement of the public concerns raised about the vaccine. Beneath it all is the unwillingness by Oxford/AstraZeneca to admit it made mistakes or that the vaccine itself is defective. The reason may not be far-fetched. Oxford/AstraZeneca has spent huge fortune (in terms of financial cost and outlay) to produce the vaccine. No company is willing to throw away such a humongous amount of cost without a fight. The company’s unwillingness is also backed up by the British political class most especially that of the Conservative Party led by Boris Johnson whose fortune is directly linked to the enourmous support enjoyed from the British Corporate World or Big Business.
The Global Race for COVID-19 Vaccine
The global race for Covid-19 vaccine is another powerful factor that could lend hand to making the mistake in the experimental laboratory at Oxford. There is no doubt that there is a global competition not only among the Big Pharma but among the powerful countries of the North.
Oxford/AstraZeneca was caught in this fierce break-neck race for vaccine especially among the three-top vaccine manufacturers: PfizerBioNTech, Moderna and Oxford/AstraZeneca to which the latter is often compared. Oxford/AstraZeneca has often rejected such comparison based on the argument that the three were working on different trajectories. This race can be seen to be a matter of life and death because prestige and reputation hang on it. Who is to cross the finishing lane first? This fierce race could have been one of the causes of the “mistake” made in the laboratory, but a cause the Oxford team is not willing to even consider and accept as a possibility.
It would be recalled that it was Russia that first announced the discovery of vaccine for the coronavirus as far back as August 2020. The Russian vaccine attracted widespread criticized for skipping the third stage of testing before allegedly administering it to its citizens. But the Russian vaccine did not attract the kind of controversy that Oxford/AstraZeneca has attracted so far.
In the West, all eyes were fixated with the ongoing Oxford/AstraZeneca efforts, including PfizerBioNTech and Moderna. Earlier, the West, especially the United States has been heavily criticized for abdicating its expected global leadership role in combating the advancing coronavirus pandemic.
It is none other small South Asian and Pacific countries (now referred to as the Mekong-Pacific Knights) led by Taiwan, Singapore, Australia, New Zealand, Vietnam, etc, that speedily halted the pandemic as early as May-June 2020 with application of variety of safety measures especially lockdowns, etc.
There was undeniable build-up of pressure to find vaccine for the coronavirus not minding the fact that vaccine has been acknowledged to take about ten years to produce after going through series of trials and safety tests. Coronavirus pandemic accelerated the pressure and all the Big Pharma companies were caught up in the whirlpool of this pressure. This inevitably have enormous impact on the work being carried out at the University
According to The Lancet editorial, “The race to develop safe, effective vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has produced impressive results. As of Jan 18, 2021, 64 vaccines were in clinical development according to the WHO COVID-19 candidate vaccine database. Phase 3 data for two RNA vaccines, BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna), and the adenovirus-vectored vaccine ChAdOx1 nCoV-19 (Oxford University/AstraZeneca) have been published in peer-reviewed journals, and further phase 3 reports are expected imminently. Although the start of mass vaccination programmes should be celebrated, many challenges lie ahead in reaching eligible recipients and protecting those at risk from COVID-19-related morbidity and mortality.”176
The UK was quick off the mark: BNT162b2 was approved for use by the Medicines and Healthcare Products Regulatory Agency on Dec 2, 2020, followed by ChAdOx1 nCoV-19 and mRNA-1273, and more than 4 million people have received a first vaccine dose. But the UK’s decision to give the second dose of BNT162b2 up to 12 weeks after the first—a delay introduced to allow more individuals to receive their first shot—has met with controversy, with Pfizer stressing that efficacy was tested with two doses given just 21 days apart. Delays in the delivery of BNT162b2 are currently affecting the UK and other European countries as Pfizer ramps up its production capacity, and limited supply of approved vaccines is likely to be an ongoing problem.177
Other countries hit hard by COVID-19 have announced ambitious plans. On Jan 16, 2021, India launched its programme with the goal of reaching 300 million people by August, 2021. The country has approved the use of ChAdOx1 nCoV-19 and the whole-virion inactivated vaccine BBV152 (Bharat Biotech); however, there are fears that the rapid approval of BBV152, phase 3 results for which are not expected until March, 2021, might undermine confidence and add to the growing problem of vaccine hesitancy in India. India has huge vaccine-manufacturing capacity and both will be produced locally, but whether the challenges of reaching priority groups can be overcome remains to be seen. For many low-income and middle-income countries, the task ahead is formidable, with concerns about the availability and affordability of vaccines. International initiatives such as the COVID-19 Vaccines Global Access (COVAX) Facility aim to promote the equitable distribution of safe and efficacious vaccines.178
Natural or vaccine-induced immunity has been a key focus of research since the start of the pandemic. Scientists worked quickly to describe the adaptive response to SARS-CoV-2 infection, including neutralising antibody and T-cell subsets. However, the duration of protection after infection is still uncertain and reinfection has been reported. The hope is that vaccines will provide lasting benefits, but careful monitoring will be needed to establish the durability of protection. Other priorities for research include the identification of immune correlates of asymptomatic or mild versus more severe disease, which could pave the way to new therapeutic approaches, and better mapping of protective epitopes to allow the fine-tuning of vaccine design and further development of therapeutic monoclonal antibodies. The long-term safety and efficacy of vaccines will need to be studied in diverse populations.179
The ability of SARS-CoV-2 vaccines to prevent infection or ongoing transmission remains unclear. The impact of immunisation on hospital admissions will be an important focus as countries aim to keep health-care systems running and protect those most at risk of severe disease in the face of high levels of transmission. Emerging SARS-CoV-2 variants are a cause for concern. New variants include B.1.1.7, which recently emerged in the UK, B.1.351, and P.1. These lineages appear to be more transmissible than previous strains, although there is currently no evidence that they cause more severe disease or compromise the efficacy of available vaccines. Surveillance is needed to detect escape variants at an early stage. New variants also emphasise the ongoing need for public health mitigation strategies and add to the urgency of vaccine rollout across the globe.180
Despite the promise of vaccination programmes and related research efforts, it will be months before the benefits start to be felt globally. In the meantime, a redoubling of efforts is needed to stem the transmission of COVID-19; to better understand the pathophysiology and clinical features of acute illness and the long-term consequences of SARS-CoV-2 infection; and to address the clinical needs of patients through evidence-based treatment and management strategies.181
However, according to Ewen Callaway, [m]ore than 90 vaccines are being developed against SARS-CoV-2 by research teams in companies and universities across the world. Researchers are trialling different technologies, some of which haven’t been used in a licensed vaccine before. At least six groups have already begun injecting formulations into volunteers in safety trials; others have started testing in animals. Nature’s graphical guide explains each vaccine design.182
The Lancet editorial did not mention what the Russians claimed to have achieved with Gamaleya Sputnik V vaccine as far back as August 2020 which went through its own controversy but not comparable with what Oxford/AstraZeneca vaccine has gone currrently gone through. The Lancet editorial did not also mention what the Chinese have done which they are now selling to those willing to buy many in the developing countries. It did not also mention Jansen, Johnson&Johnson and numerous others.
Was EU countries justified in rejecting the vaccine?
The question has been raised as to whether the decision by several European countries to suspend the use of Oxford/AstraZeneca vaccine is a case of being too cautious. Are these nations missing the bigger picture?
There may probably be no doubt about this. However, in the context of geopolitical considerations, caution can easily be overthrown in favour of seeking vengeance for the very pandemonium that Brexit threw the European Union which forms part of the unfinished business
According to Nick Triggle (2021): “The decision has been made on the basis of the precautionary principle – a well-established approach in science and medicine that stresses the need to pause and review when evidence is uncertain.”183 But in a fast-moving pandemic, when each decision can have major consequences, it is an approach which can sometimes do more harm than good.184.
The data supplied by AstraZeneca shows there have been 37 reports of blood clots among the 17m people across Europe who have been given the vaccine. But the key question that has to be asked is whether this is cause or coincidence? Would these clots have happened anyway? Adverse events like this are monitored carefully, so regulators can assess if they are happening more than they should.185
The 37 reports of clots are below the level you would expect. What is more, there is no strong biological explanation why the vaccine would cause a blood clot. It is why the World Health Organization and UK drugs regulators have all said there is no evidence of a link.186
Even the European Medicines Agency, which is looking into the reports, has suggested the vaccine should continue to be used in the meantime given the risk Covid presents to health. Unsurprisingly, therefore, the decisions by individual nations to pause their rollouts have baffled experts.187
Prof Adam Finn, a member of the WHO’s working group on Covid vaccines, says stopping rollout in this way is “highly undesirable” and could undermine confidence in the vaccine, costing lives in the long-term. “Making the right call in situations like this is not easy, but having a steady hand on the tiller is probably what is needed most.”188
This is not the first time countries in Europe have exercised caution about the AstraZeneca vaccine. The precautionary principle was adopted by Germany, France and others when they did not initially recommend use of the vaccine for the over-65s. French President Emmanuel Macron even called it “quasi-ineffective”. The over 65s decision has now been reversed, but the impact is still being felt it seems. Germany and France have supplies of the vaccine going to waste, with both countries having used fewer than half their supplies of the AstraZeneca jab so far. It has left them far more reliant on the Pfizer vaccine than the UK is. And this is threatening to have deadly consequences. France, Germany and the other major European nations all have higher rates of infection than the UK, and face the prospect of things getting worse before they get better.189
If you look back at the decision on the over-65s, you can see how it was made. The way the trials had been organised meant there was limited evidence on its use in older age groups. The organisers had wanted to recruit younger adults in the initial stages for safety reasons, so when it came to regulators assessing infection rates the data was not yet ready for older people.190
But there was evidence from blood samples that the vaccine had prompted a strong immune response in the older age groups. So there was no reason why the vaccine would not work in older age groups – it was just that insufficient time had passed to gather the evidence in the real world. There was also a problem interpreting results because of the way the trial was run. There was a lack of consistency across the different sites used. Protocols and practices followed varied across each, including the use of a half-dose in one. Some have described it as four trials within a trial. It created a somewhat messy set of data to interpret.191
The Pfizer vaccine was not tested like this in trials – the interval was three weeks. But again, the absence of evidence did not mean the move would not work or was not based on logic. The AstraZeneca trials did have longer intervals for some participants, which seemed to make it more effective, while evidence on the Moderna vaccine, which is a similar type of jab to Pfizer, also suggested it could work. It is also well-established that with two-dose vaccines, most of the protection is provided by the first dose, while the second boosts that and provides more long-lasting protection. With cases rising rapidly at the time, the UK was clear the benefit of maximising available vaccine supplies to provide some protection to more people was the right step even if the trial evidence did not directly support it.192
Prof Sir David Spiegelhalter, an expert in understanding risk at Cambridge University, says it shows that sometimes you have to look beyond the precautionary principle and be bold in your decision-making. “The precautionary principle favours inaction as a way of reducing risk. But the problem is that these are not normal times and inaction can be more risky than action.” What is needed in circumstances like these, according to Sir David, is to work out what is most likely on the balance of probability. That requires looking at both the direct and indirect evidence and the context those decisions are being made in. “Sometimes it can be harmful to wait for certainty. Not vaccinating people will costs lives.”193
It is the second time EU countries have departed from the advice of the bloc’s medicines regulator on the Oxford/AstraZeneca vaccine.194
Oxford/AstraZeneca vaccine also faces problem of supply. It reneged on its delivery promise to supply 40 million doses in the first quarter and reduced it to 30 million doses while the EU is pressing for even higher number.
After a very public back-and-forth over unexpectedly tight COVID-19 vaccine supplies in Europe earlier this year, AstraZeneca said it would aim to deliver 40 million doses in the first quarter.195 Now, it’s rolling the estimate back to 30 million doses, Reuters reports —about the same number that triggered the initial controversy. The company confirmed the shortfall in a statement on Friday.195
AstraZeneca originally committed to delivering 90 million doses to the continent during the first three months of the year, Reuters reports, but has run into problems getting its supply chain up to speed. In January, the company cut its delivery target to 31 million—and an outcry ensued.196
CEO Pascal Soriot went on the defense, but European officials were adamant. Eventually, AZ said it would push to deliver 40 million doses. But now that target seems out of reach. AstraZeneca now plans to deliver 30 million doses in the first quarter, and 100 million doses in the first half of the year, the company said. Export restrictions will likely affect its second-quarter deliveries, the company added.197
Soriot said last month the company is using a global manufacturing network, and some sites are farther along than others in learning how to scale up the complex manufacturing process. The company’s European network has run into problems with yield, according to reports.198
AstraZeneca’s vaccine is one of four approved shots in Europe, but the bloc still lags the global leaders in the worldwide vaccine push by a significant margin. This comes as more than half of the AstraZeneca vaccines doses in Germany, France, Italy and Poland have gone unused, according to data from the European Centre for Disease Prevention and Control.199
The travail of Oxford/AstraZeneca is not limited to the supply chain hiccups alone. Apart from problems associated with supply chain i.e. the inability to meet market demand, AstraZeneca [said] that its vaccine is 70 percent effective, but a major dosing error that occurred during the trials may have impacted the overall efficacy.200 Some participants accidentally received a weakened dose, but ended up producing a more robust immune response. Though the mistake led to an important discovery, some health experts would like to see AstraZeneca and regulators further investigate the dosing mishap to determine the vaccine’s true safety and efficacy.201
The 70% efficacy rate was also another source of controversy and headache for Oxford/AstraZeneca
AstraZeneca’s COVID-19 vaccine has achieved 70% efficacy in a late-phase analysis. The 70% figure is an average that masks variation in the performance of two dosing regimens, one of which was found to be 90% effective.202
With the Pfizer-BioNTech and Moderna vaccines achieving efficacy of around 94%, the bar was set high for AstraZeneca’s AZD1222. Overall, the COVID-19 vaccine, which AstraZeneca is developing with the University of Oxford, fell well short of that bar. Yet, the details offer encouragement that the vaccine can be highly effective and more broadly adopted than the other candidates.203
The 70% efficacy figure is the average result for two regimens given to 11,636 people. Most subjects, 8,895, received the same dose for their first and second jabs. A smaller cohort, 2,741 subjects, got a half dose for the first jab followed by a full booster shot.204
Vaccine efficacy in the cohort of subjects who received two full doses was 62%. Efficacy in subjects who began with a half dose was 90%. Nobody who received the vaccine was hospitalized or had a severe case of COVID-19. The difference between the two regimens may reflect AstraZeneca’s use of a chimpanzee viral vector to deliver the payload of the vaccine.205
“One potential explanation for this is that the body is mounting an anti-vector immune response to the second dose, which is lower if the first dose is reduced; such an immune reaction could reduce the efficacy of the vaccine,” Gillies O’Bryan-Tear, chair of policy and communications for the Faculty of Pharmaceutical Medicine, said in a statement.206
The relatively small number of subjects enrolled in the half-dose cohort makes it hard to draw firm conclusions about the finding. Further use of the regimen should show whether 90% is an accurate reflection of the effectiveness of the half-dose approach. The disclosure sparked criticism.207
“The company tried to embellish their results by highlighting a reported 90% efficacy in a relatively small sub-set of subjects in the study. They did not disclose the exact number of events in each study, or how they calculated the blended 70% efficacy for the full cohort. The suggestion by the inventors that the small sample given the lower priming dose was evidence of superior efficacy only brings discredit to the program. We regard the data disclosure as premature and insufficient,” SVB Leerink analyst Geoffrey Porges wrote in a note to investors.208
Differences between the designs of the clinical trials of different vaccines may have contributed to the seemingly lower efficacy of AstraZeneca’s candidate. Notably, AstraZeneca’s U.K. trial swabbed participants weekly, positioning it to detect asymptomatic cases. Pfizer and Moderna’s results are based on symptomatic cases.209
Safety was a concern during the development of AZD1222, with an adverse event putting the R&D program on pause at one point. AstraZeneca is yet to share late-phase safety and tolerability data but said no serious safety events related to the vaccine have been confirmed. Some observers were critical of the level of disclosure.210
“The company is likely to be roundly criticized today for their disclosure, since the safety disclosure simply state that “no serious safety events related to the vaccine have been confirmed” which is hardly reassuring. They did not disclose any information about any actual safety events,” Porges wrote.211
With these types of sentiments, looking exclusively at the alleged shortcomings of the vaccine without looking at the overall environment of hidden political calculations which may not be expressed publicly, it is easy to jump into conclusion that the decision to suspend the use of the vaccine by these countries is over-cautiousness, ill-motivated and misplaced.
Indeed these are lamentation of the injured feelings or wounded pride to have vaccine from the biggest vaccine manufacturer in the United Kingdom rejected peremptorily. The European countries are definitely not unaware of the fine details (which probably could have been sorted out with the manufacturer in the boardroom without taking to the public domain). But to have turned blind eyes to these details shows clearly that the decision to reject the vaccine is not based on those details but on political considerations – of course to which they are entitled to protect their perceived public health safety or national security interests.
Of course, irreparable damage has been done to the British prestige even if the decision is later reversed. The decision only shows they can hurt Britain not minding whether the same decision also or can hurt their own citizens suffering from the coronavirus pandemic.
Another report speculated that “Asia’s economic recovery could slow down as more countries suspend the use of the Covid-19 vaccine developed by AstraZeneca and the University of Oxford, warned the chief Asia-Pacific economist of Moody’s Analytics.” “It adds some modest risk to the role that Asia plays in terms of the global economic turnaround,” Steve Cochrane told CNBC’s “Squawk Box Asia” on Tuesday.212
“The vaccine is a risk, of course. It’s one of the critical risks, we still have to see vaccines roll out over the course of this year for the global economy to get back on its feet”, [said Steve Cochrane, Chief Asia-Pacific Economist, Moody’s Analytics]. Cochrane said issues surrounding the AstraZeneca-Oxford vaccine could hurt global trade — and that’s bad news for Asia where many economies are dependent on trading activity.“There’s a possibility it could put a dent in terms of global trade if the vaccine rollout is delayed in Europe and that were to mean that there were some more extensive lockdowns on the economy in Europe — then that could slow down the pace of global trade,” he explained.213
Asian countries have been relatively successful in containing the virus, and that’s helped their economies recover quicker than those in Europe and the U.S. Fortunately, renewed lockdowns in some parts of Europe have not hit manufacturing, said Cochrane. He added that “almost all” of the impact from those lockdowns have affected the services sector. “So, right now, it’s not that big of an issue and global trade still looks like it’s very, very strong,” said the economist. “The vaccine is a risk, of course. It’s one of the critical risks, we still have to see vaccines roll out over the course of this year for the global economy to get back on its feet.”214
Thailand briefly halted the use of the AstraZeneca-Oxford vaccine on Friday, but authorities said on Monday they would go ahead with administering the shots. The Prime Minister became the first person in the country to receive the AstraZeneca-Oxford shot on Tuesday, reported Reuters. Elsewhere in Asia, Indonesia said Monday it will delay the rollout of the AstraZeneca-Oxford vaccine while waiting for the review from WHO, the news agency reported.215
This is red herring. The logical sense does not flow from the analysis by Moody.
It is not only Thailand that refused to use the Oxford/AstraZeneca vaccine outside Europe. Venezuela in faraway South America is another example that showed hostility towards the vaccine. “Venezuela is not too friendly to Britain at any rate. “Venezuela will not grant a license to use the AstraZeneca vaccine due to “adverse results” on people who received the vaccine, the Foreign Ministry has said, quoting Vice President Delcy Rodriguez.”216
Where is Britain with Vaccination?
The battle against the pandemic in the United Kingdom can be gleaned from the various exercises carried out by the relevant statutory bodies.
More than 20 million people across the United Kingdom have now received their first COVID-19 vaccine shot, data showed on Sunday as the country made more progress with Europe’s fastest vaccination programme.217
Prime Minister Boris Johnson said the milestone represented “a huge national achievement and he paid testament to the country’s health, workers, volunteers and armed forces. “I urge everyone to get the jab when called,” Johnson said. “Every jab makes a difference in our battle against COVID.”218
Britain has suffered the highest COVID-19 death toll in Europe – it currently stands at 122,849 – and the heaviest economic shock among big rich countries, according to the headline measures of official data. But the pace of its vaccination roll-out has raised the prospect of a gradual lifting of its current lockdown restrictions between now and the end of June.219
On Sunday, finance minister Rishi Sunak promised to help the economy while the country remains under restrictions. In a budget statement on Wednesday, he is expected to announce more borrowing on top of his almost 300 billion pounds ($418 billion) of COVID-19 spending and tax cuts.220
Official data showed a total of 20.09 million people in Britain have received their first dose of a COVID-19 vaccine and almost 800,000 have received a second dose. …Britain said more than one in three adults had received their first vaccination. Britain also reported a further 6,035 cases within the previous 24 hours, and 144 more deaths within 28 days of a positive test. The latest figures meant cases over the past seven days were down 21.2% compared with the previous seven-day period of Feb. 15-21, and deaths were down 33.5%.221
As of February 28, 2021, approximately 20.2 million people in the United Kingdom had received the first dose of the COVID-19 vaccination. The UK was the first country in the world to approve the use of the PfizerBioNTech vaccine and began inoculations on December 8. The vaccine requires two doses taken around three weeks apart for full efficacy to occur, and according to the latest data around 814 thousand people had received their second dose of the immunization.222
As at March 16 2021, virus tests conducted on daily basis stands at 1,573,774 with a PCR testing capacity at 734,609 while people vaccinated with thefirst dose total standing at 24,839,906 and with the second dose total standing at 1,663,646.223
According to Wikipedia the COVID-19 vaccination programme in the United Kingdom is the world’s first mass immunization campaign to protect against SARS-CoV-2 using vaccines developed in response to the COVID-19 pandemic.224
Vaccinations began on 8 December 2020, shortly after the British regulator, the Medicines and Healthcare products Regulatory Agency (MHRA), granted emergency authorisation to the PfizerBioNTech vaccine, an RNA vaccine. On 30 December 2020, the MHRA gave approval to a second vaccine, the OxfordAstraZeneca, and vaccinations began on 4 January 2021, with a third vaccine, the Moderna vaccine, approved on 8 January 2021.225
The two vaccines currently in use were developed by partnerships between Pfizer and BioNTech (Cominaty), and the University of Oxford and AstraZeneca (AZD1222). A third, approved but not yet being administered, was developed by Moderna (MRNA-1273). As of 7 February 2021, there were five other COVID-19 vaccines on order for the programme, at varying stages of development.226
Phase 1 of the rollout prioritises the most vulnerable, in a schedule primarily based on age. The delivery plan was adjusted on 30 December 2020, delaying second doses so that more people could receive their first dose. A preliminary target – giving everyone in the top four priority groups their first dose by the middle of February 2021 – was announced on 4 January 2021.227
As of 11 March 2021: 23,053,716 first doses and 1,351,515 second doses, of either the Oxford–AstraZeneca or the Pfizer–BioNTech vaccine, had been administered across the UK.228
Vaccination sites include GP practices, care homes and pharmacies, as well as hospitals. As of 29 January 2021, there were 1,462 vaccination sites operating in England. As of 2 February, there were over 400 vaccination sites in Wales. Additional sites, including large venues such as sports stadia, entered the programme from 11 January 2021, with seven mass vaccination centres opening in England initially and seven opening in Wales also.229
The government has announced a target to offer first doses to 15 million people in the top four priority groups by 15 February 2021, this target was met on 14 February 2021. Prime Minister Boris Johnson, subsequently announced the next 5 groups will be offered a vaccine by 15th April. This means 32 million vaccines will have been administered by the middle of April 2021.230
Despite the snowballing controversy, as of March 16, 2021, [m]ore than 24 million people in the UK have received at least one dose of a coronavirus vaccine – part of the biggest inoculation programme the country has ever launched.231 In a race against a faster-spreading variant of the virus, ministers have pinned their hopes of easing a third national lockdown on vaccinating as many adults as possible by summer.232
More than 24 million people so far have had a first vaccine dose and about 1.6 million have had a second. The number of first doses administered each day had been steadily climbing since December – reaching more than 400,000 a day mid-February. However, the current seven-day average for first doses is about 300,000 doses a day. Last week, the UK’s vaccines minister Nadhim Zahawi told MPs that although supply had been “finite” until now, the country would see a “big uplift” in available doses in the second half of March – amounting to tens of millions of jabs.
NHS sources suggest the number of shots given is expected to top 4 million this week.233
The campaign to reach as many people as quickly as possible was boosted by a shift in policy in early January – to prioritise the first dose of a vaccine, with a second dose up to 12 weeks later, a bigger gap than originally planned. The progress made in the UK so far means the country continues to be among those with the highest vaccination rates globally.234
Are the vaccines having an impact?
When looking for evidence for whether the campaign is working, data for England shows coronavirus numbers are falling faster for vaccinated groups compared with unvaccinated groups. This suggests that the vaccine is starting to push numbers down, instead of as a result other factors, like the lockdown.235
On average, deaths of over-65s fell by 63% between 19 February-5 March, compared with 53% for under-65s. Coronavirus hospital admissions are also falling faster for older age groups.236
Another critical question is whether there is enough vaccine within the context of the Oxford/AstraZeneca problematique
The UK has ordered more than 400 million doses of seven of the most promising vaccines. Three have so far been approved for use: Oxford-AstraZeneca; Pfizer-BioNTech; and Moderna.237
The UK government has also announced an eighth deal with biopharmaceutical company CureVac to develop vaccines against future variants. It has placed an initial order for 50 million doses to be delivered later this year – if they are required. But there have been a number of challenges in what is called the vaccine “supply chain” – the logistics of how the jab gets from manufacturers to people. Getting enough supplies, checking those supplies are up to scratch and transporting vaccines according to their requirements have all thrown up difficulties.238
The Unknown Politics of WHO
It is interesting to note that while several European countries are rejecting the Oxford/AstraZeneca vaccine, the World Health Organization was urging the Brits and the rest of the world that has ordered the vaccine to continue to use it. This would not have been a subject of criticism had it not been for the profile of failure of the body in the past and present as regard management of the coronavirus pandemic.
This may probably not be surprising if the WHO’s previous dirigisme and mindset are taken into consideration. WHO’s archetypal worldview/dirigisme and operative mindset have been the support of officialdom, This has come to reach its apogee under the current Director General, Dr.Tedro Adhamon Grebeyesus: playing safe and never rock the boat! Or no permanent friends, no permanent enemies but permanent interest of staying in power even amidst moral opprobrium or condemnation for vacillations or pusillanimity!
WHO obviously do not want to rock the boat. It wants to play safe or wait to see where the wind is blowing before safely coming out but timidly to offer criticisms or advisories which it could have done earlier to save the world the headache of confusion caused by WHO’s often-ambivalent positions on critical issues.
WHO did the same thing with China until it could no longer continue to do so. WHO was in defense of China until it became too apparent that it was not wise to do so anymore. WHO could not criticize China for anything until China denied visas to its officials scheduled to go to China for further investigations about the origin of the oubtbreak of the coronavirus in January 2021. Eventually when WHO officials entered China after much wrangling, it still came back empty handed.
The World Health Organization squarely endorsed AstraZeneca coronavirus vaccine on Friday, as Thailand joined a number of smaller European countries in suspending use of the shot because of sporadic reports of blood clots among recipients. Bulgaria also joined Denmark, Norway and Iceland, which all stopped using the vaccine on Thursday. Austria, Italy, Luxembourg, Estonia, Lithuania and Latvia stopped using certain batches. “Until all doubts are dispelled…, we are halting inoculations with this vaccine,” Bulgarian Prime Minister Boyko Borissov said.239
His health minister, Kostadin Angelov, said a 57-year-old woman had died of heart failure 15 hours after receiving an AstraZeneca shot, but urged those already inoculated to stay calm. “We do not have any official data that proves a causal connection,” he said. That line was reinforced by the WHO, which is keenly aware that AstraZeneca’s shot is by far the cheapest and most high-volume launched so far, and set to be the mainstay of vaccination programs in much of the developing world. Spokeswoman Margaret Harris said the vaccine was “excellent.”240
Experts point to the difficulty of putting risks in perspective for a wider public that may be spooked by negative headlines. In Sicily, where two people died shortly after being vaccinated, the regional health administrator said 7,000 inoculation appointments had been canceled as a result. Silvestro Scotti, a family doctor in Naples and head of the Italian Federation of General Practitioners, said he had been bombarded all day with inquiries from people nervous about getting the AstraZeneca shot. “The crazy thing is that, even if the correlation between the vaccine and blood clots were proved, it would be a rate of 0.007 out of a thousand,” he said. “To give an example: the birth control pill, which is used widely and doesn’t worry anyone, has a proven risk rate of 0.6 in a thousand. Even in the worst-case scenario, the risk/benefit ratio for this vaccine is extraordinarily favorable. That needs to be explained to people.”241
The WHO’s Harris said 268 million doses of COVID-19 vaccines from various developers had been administered worldwide without being shown to have caused a single death.
In France, where distrust of vaccination is long-established, only 43 per cent said they trusted the AstraZeneca shot in a Harris Interactive poll conducted on March 11-12, while 55 per cent said they trusted COVID-19 vaccines in general.Germany has also had to contend with substantial skepticism, to the extent that Health Minister Jens Spahn suggested that the AstraZeneca shot be given to the police force and army, after some health and other frontline workers balked at receiving it. However, German authorities’ main concern has been lack of supply, rather than lack of acceptance, as social and economic restrictions to limit transmission take their toll. One doctor administering vaccinations in Berlin said recipients were now asking far fewer questions about the vaccine than two weeks ago.242
Health Canada said Thursday it will move forward with administering the Oxford-AstraZeneca COVID-19 vaccine despite at least nine European countries stopping its use. Health Canada spokesperson Tammy Jarbeau told Global News the agency is aware of reports of adverse events in Europe and would “like to reassure Canadians that the benefits of the vaccine continue to outweigh its risks.” “Health Canada authorized the vaccine based on a thorough, independent review of the evidence and determined that it meets Canada’s stringent safety, efficacy and quality requirements,” Jarbeau said.243
One may not understand WHO’s position without considering the works it has done in respect of the vaccine. In short, WHO is morbidly afraid of consigning all the works it has done to the waste basket if it should join the legion of critics of the Oxford/AstraZeneca vaccine in the last few months. Interestingly WHO is probably not unaware of the gaps in knowledge that the Oxford/AstraZeneca vaccine have generated in the public domain.
In mid-September 2020 and in anticipation of manufacture of vaccine fot the coronavirus and its roll-out for vaccination, the Strategic Advisory Group of Experts (SAGE) on Immunization of the World Health Organization (WHO) developed a Values Framework that offers global guidance for the allocation of Covid-19 vaccines between countries and a prioritization of groups within countries in context of limited supply. “This Values Framework offers guidance globally on the allocation of COVID-19 vaccines between countries, and to offer guidance nationally on the prioritization of groups for vaccination within countries while supply is limited. The Framework is intended to be helpful to policy makers and expert advisors at the global, regional and national level as they make allocation and prioritization decisions about COVID-19 vaccines. This document has been endorsed by the Strategic Advisory Group of Experts on Immunization (SAGE).244
The Framework articulates the overall goal of COVID19 vaccine deployment, provides six core principles that should guide distribution and twelve objectives that further specify the six principles. To provide recommendations for allocating vaccines between countries and prioritizing groups for vaccination within each country, the Values Framework needs to be complemented with information about specific characteristics of available vaccine or vaccines, the benefit-risk assessment for different population groups, the amount and pace of vaccine supply, and the current state of the epidemiology, clinical management, and economic and social impact of the pandemic. Hence, the final vaccination strategy will be defined by the characteristics of vaccine products as they become available.245
SAGE is currently engaged in the process of applying the Values Framework to emerging evidence on specific vaccines, and the evolving epidemiology and economic impact of the pandemic. The first stage of this process was the identification of populations and sub-populations which would be appropriate target groups for prioritization under the various values-based objectives in the Framework, before data on Phase 3 vaccine performance are not yet available. Specific priority group recommendations for specific vaccines will be made as vaccine products become authorized for use; initial vaccine specific policy recommendations are expected in the final quarter of 2020 or early 2021, depending on timing of and findings from phase 3 vaccine trials.246
The Framework also complements the principles on equitable access and fair allocation of COVID-19 health products developed for the ACT Accelerator COVAX facility.247
The framework goals and principles are as follows:
- Overarching Goal: COVID-19 vaccines must be a global public good. The overarching goal is for COVID-19 vaccines to contribute significantly to the equitable protection and promotion of human well-being among all people of the world.
Principles
- Human Well-Being: Protect and promote human well-being including health, social and economic security, human rights and civil liberties, and child development.
- Equal Respect: Recognize and treat all human beings as having equal moral status and their interests as deserving of equal moral consideration.
- Global Equity: Ensure equity in vaccine access and benefit globally among people living in all countries, particularly those living in low-and middle-income countries.
- National Equity: Ensure equity in vaccine access and benefit within countries for groups experiencing greater burdens from the COVID-19 pandemic.
- Reciprocity: Honor obligations of reciprocity to those individuals and groups within countries who bear significant additional risks and burdens of COVID-19 response for the benefit of society.
- Legitimacy: Make global decisions about vaccine allocation and national decisions about vaccine prioritization through transparent processes that are based on shared values, best available scientific evidence, and appropriate representation and input by affected parties.248
Around mid-November 2020, the Strategic Advisory Group of Experts (SAGE) on Immunization of the World Health Organization (WHO) published a roadmap for prioritizing uses of Covid-19 Vaccines in the context of limited supply.
As countries prepare to implement their respective coronavirus disease 2019 (COVID-19) vaccination programmes, the Strategic Advisory Group of Experts (SAGE) on Immunization of the World Health Organization (WHO) is undertaking a three-step process to provide guidance for overall programme strategy as well as vaccine-specific recommendations.249
Step 1: A Values Framework. The WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination (1), issued on 14 September 2020, outlines the general principles, objectives and related (unranked) target groups for prioritization of COVID-19 vaccines.
Step 2: Roadmap for prioritizing uses of Covid-19 vaccines (Prioritization Roadmap) (this document). To support countries in planning, the Roadmap suggests public health strategies and target priority groups for different levels of vaccine availability and epidemiologic settings. The Roadmap will be updated, as necessary, to accommodate the dynamic nature of the pandemic and evolving evidence about vaccine impact.
Step 3: Vaccine-specific recommendations. As market-authorized vaccines become available, specific recommendations for the use of these vaccines will be issued. These recommendations may be updated as additional evidence of effectiveness and safety of market-authorized vaccines (as well as other interventions) becomes available and as epidemiologic and other contextual conditions evolve.
Given the urgency and wide-ranging effects of the COVID-19 pandemic, SAGE has developed an approach to help inform deliberation around the range of recommendations that may be appropriate under different epidemiologic and vaccine supply conditions. The SAGE consensus is that currently available evidence is too limited to allow any recommendations for use of any specific vaccine against COVID-19 at this time (7 October 2020). This document should be regarded as a Roadmap for planning purposes only.250
This Roadmap builds on the WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination. The Values Framework listed over 20 population subgroups that, if vaccine use needed to be prioritized because of limited supply, would advance one or more of its principles and objectives. The Values Framework did not rank the subgroups in any order. Specific priority group recommendations for each vaccine product as it becomes authorized for use will require the integration of these ethical principles detailed in the Values Framework with evidence and information about: i) the status of the pandemic in the proposed implementation area (that is, the epidemiologic setting in terms of the degree of ongoing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission and COVID-19 burden); ii) the amount and timing of vaccine supply and availability, respectively; iii) specific product characteristics of the available vaccine(s); and iv) the benefit–risk assessment for the different population subgroups at the time vaccination is being considered for deployment; as well as other standard criteria used in developing SAGE recommendations (for example, feasibility, acceptability and resource use). These factors, together with the Values Framework, should guide the appropriate public health strategy for vaccine deployment of specific vaccines.251
To assist in developing recommendations for use of vaccines against COVID-19, SAGE proposes a Prioritization Roadmap of COVID-19 vaccines that considers priority groups for vaccination based on epidemiologic setting and vaccine supply scenarios. These use cases are also set in the context of the overall public health strategy for each epidemiologic setting.252
This Roadmap is intended to serve as guidance on preparing for vaccine prioritization decisions within countries. Although the Values Framework does include the principle of global equity, this Roadmap does not directly address global allocation decisions. A COVAX Facility allocation mechanism for countries participating in the COVAX Facility has been proposed.253
WHO also published a set of interim recommendations applying to the Oxford/AstraZeneca vaccine.
These interim recommendations apply to AZD1222 (ChAdOx1-S [recombinant]) vaccine against COVID-19 developed by Oxford University (United Kingdom) and AstraZeneca as well as to ChAdOx1-S [recombinant] vaccines against COVID-19 produced by other manufacturers that rely on the AstraZeneca core clinical data, following demonstrated equivalence in their regulatory review and once emergency use listing (EUL) has been obtained from WHO.254
The guidance is based on the evidence summarized in the Background document on AZD1222 vaccine against COVID-19 developed by Oxford University and AstraZeneca and the Background paper on COVID-19 disease and vaccines. Both these documents are available on the SAGE COVID-19 webpage: https://www.who.int/groups/strategic-advisory-group-of-experts-onimmunization/covid-19-materials. 255
The COVID-19 pandemic has caused significant morbidity and mortality throughout the world, as well as major social, educational and economic disruptions. There is an urgent global need to develop effective and safe vaccines and to make them available at scale and equitably across all countries.256
The AZD1222 vaccine against COVID-19 has an efficacy of 63.09% (95% CI 51.81; 71.73) against symptomatic SARS-CoV-2 infection, as shown by the primary analysis of data irrespective of interdose interval (data cut 7 December 2020) from trial participants in the United Kingdom, Brazil and South Africa who received 2 standard doses. Vaccine efficacy tended to be higher when the interval between doses was longer. This, together with the finding of higher antibody levels with increasing interdose interval, supports the conclusion that longer dose intervals within the 4–12 weeks range are associated with greater vaccine efficacy. No vaccinated persons were hospitalized as from 22 days after dose 1, compared with 14 unvaccinated persons who were hospitalized for COVID-19 in the same time frame. At the time of analysis, the median follow-up time after the second dose was 80 days. More detailed data on the efficacy and safety of this vaccine can be found in the Background document on AZD1222 vaccine against COVID-19 developed by Oxford University and AstraZeneca (https://www.who.int/groups/strategic-advisorygroup-of-experts-on-immunization/covid-19-materials). The data reviewed by WHO support the conclusion that the known and potential benefits of AZD1222 outweigh the known and potential risks. As sufficient vaccine supply will not be immediately available to immunize all who could benefit from it, countries are recommended to use the WHO Prioritization Roadmap (4) and the WHO Values Framework (5) as guidance for their prioritization of target groups. As long as vaccine supplies are very limited (stage I in the WHO Prioritization Roadmap), in settings with community transmission, the Roadmap recommends that priority be given initially to health workers and older people with and without comorbidities. Protecting health workers has a threefold purpose: (i) to protect the individual health workers; (ii) to protect critical essential services during the COVID-19 pandemic; and (iii) to prevent onward transmission to vulnerable people. Protecting older people will have the greatest public health impact in terms of reducing the number of deaths. As more vaccine becomes available, additional priority groups should be vaccinated as outlined in the WHO Prioritization Roadmap (4), taking into account national epidemiological data, vaccine-specific characteristics as outlined in product information approved by regulatory authorities, and other relevant considerations.257
Recommendations on addressing current knowledge gaps through further research WHO recommends the following post-authorization monitoring activities and research.
- Safety surveillance and monitoring:
− serious adverse events, anaphylaxis and other serious allergic reactions, Bell`s palsy, transverse myelitis, cases of multisystem inflammatory syndrome following vaccination, cases of COVID-19 following vaccination that result in hospitalization or death;
− background rates of AESIs, maternal and neonatal outcomes, and mortality in groups prioritized for vaccination.
- Vaccine effectiveness:
− vaccine effectiveness in older persons; − vaccine effectiveness in relation to time interval between the first and second dose;
− vaccine effectiveness in relation to new virus variants; − vaccine effectiveness over time and whether protection can be prolonged by booster doses;
− booster studies with heterologous vaccines;
− studies to investigate whether this vaccine reduces SARS-CoV-2 transmission and viral shedding;
− assessment and reporting of breakthrough infections and virus sequence information;
− head-to-head studies with other vaccines on extent and duration of immunity using standardized neutralization, T-cell and mucosal immunity assays.
- Subpopulations:
− prospective studies on the safety of AZD1222 vaccine in pregnant and lactating women;
− randomized controlled trials on efficacy and safety of vaccination in persons below the age of 18 years;
− safety data on vaccination in immunocompromised persons, including persons living with HIV and persons with autoimmune disease.
- Vaccination logistics
− immunogenicity and safety studies of co-administration with other vaccines, including influenza and pneumococcal vaccines, to adults and older persons;
− safety, immunogenicity, and impact of a delayed second dose, as currently implemented by certain countries;
− interchangeability and “mix and match” studies within and across COVID-19 vaccine platforms;
− stability of vaccine under alternative cold-chain distribution and storage conditions.
- Virus variants
− global surveillance of virus evolution and the impact of virus variants on vaccine effectiveness to support update of vaccines;
− Modelling to determine the trade-offs for the use of vaccines with reduced effectiveness against emergent variants;
− Booster studies with updated vaccine formulations.258
On March 1, 2021, WHO published a background document that served as basis discussions at its previous internal meetings in furtherance of the roll-out of vaccination. The document was based exclusively on previous scientific publications on the vaccine produced by Oxford/AstraZeneca.
This background document has been prepared by the Strategic Advisory Group of Experts (SAGE) on Immunization Working Group on COVID-19 Vaccines to inform the discussions of SAGE at its 8 February 2021 extraordinary meeting, which resulted in the issuance of the 10 February 2021 WHO Interim recommendations for use of the AZD1222 (ChAdOx1-S [recombinant]) vaccine against COVID19 developed by Oxford University and AstraZeneca. Both recommendations and background document are available on the SAGE Covid-19 webpage: https://www.who.int/groups/strategic-advisory-group-of-experts-on-immunization/covid-19-materials. 259
The document noted the context which informed the decision approving the vaccine: Replication-deficient adenovirus vectors containing a pathogen-specific transgene have been used as novel vaccines because of their ability to induce strong humoral and cellular responses. However, pre-existing immunity might reduce the immunogenicity of vectors derived from human viruses, and so use of simian adenoviruses might be preferable. COVID-19 Vaccine AstraZeneca, also known as AZD1222 or ChAdOx1-S (recombinant), was developed by Oxford University, United Kingdom, and AstraZeneca, and is a replication-deficient chimpanzee adenovirus-vectored vaccine expressing the full-length SARS CoV-2 spike glycoprotein gene.260
AZD1222 vaccine is a monovalent vaccine composed of a single recombinant, replication-deficient chimpanzee adenovirus vector encoding the S glycoprotein of SARS-CoV-2 (ChAdOx1-S (recombinant). The SARS-CoV-2 S immunogen in the vaccine is expressed in the trimeric prefusion conformation; the coding sequence has not been modified, in order to stabilize the expressed S-protein in the prefusion conformation. Adenoviruses are non-encapsulated, icosahedral particles (virions), and contain a single copy of the double-stranded DNA genome. The expression cassette for the SARS-CoV-2 spike protein fused to the tissue plasminogen activator leader sequence uses a modified human cytomegalovirus promoter and a bovine growth hormone polyadenylation sequence. The following information is derived from the product information approved by the European Medicine Agency`s Committee for Medicinal Products for Human Use (CHMP).261
Its preclinical studies are derived from prior scientific publications.
The efficacy of AZD1222 vaccine was assessed in rhesus macaque monkeys (2). Six animals per group were vaccinated intramuscularly with 2.5 × 1010 ChAdOx1-S (recombinant) virus particles each, using either a prime-only regimen (28 days before challenge) or a prime–boost regimen (56 and 28 days before challenge). As a control, six animals were vaccinated via the same route with the same dose of ChAdOx1-S (recombinant) green fluorescent protein (GFP) (one animal was vaccinated 56 and 28 days before challenge and five animals were vaccinated 28 days before challenge). No adverse events were observed after vaccination. Spike-specific antibodies were present as early as 14 days after vaccination and were significantly increased after the second vaccination (two-tailed signed-rank Wilcoxon test). Endpoint IgG titres of 400–6400 (prime) and 400–19 200 (prime–boost) were measured on the day of challenge. Virusspecific neutralizing antibodies were also significantly increased after the second vaccination (two-tailed signed-rank Wilcoxon test) and were detectable in all vaccinated animals before challenge (titres 5–40 (prime) and 10–160 (prime– boost)). No virus-specific neutralizing antibodies were detected in control animals. On the day of challenge, IgM antibodies were present in the serum of all six prime–boost animals and two of the six prime-only animals. SARS-CoV2 spike-specific T-cell responses were detected on the day of challenge by gamma interferon (IFNγ) ELISpot assay, after stimulation of peripheral blood mononuclear cells with a peptide library that spanned the full length of the spike protein. No statistically significant difference in the magnitude of the response was found between the prime–boost and primeonly group (Mann–Whitney U-test, P = 0.3723). Vaccination with ChAdOx1-S (recombinant) has been shown to induce neutralizing antibodies against the vaccine vector itself within 28 days of vaccination. Nonetheless, a boost vaccination with ChAdOx1-S (recombinant) resulted in a significant increase in binding and neutralizing antibodies and an increase in the SARS-CoV-2 virus-neutralizing titre was not significantly correlated with the ChAdOx1-S (recombinant) virusneutralizing titre (two-tailed Pearson correlation, r2 = 0.6493 P = 0.0529).262
After challenge, the animals were evaluated for the protection offered by the vaccine and the potential for vaccine associated enhanced respiratory disease (VAERD) (2). The clinical disease score in vaccinated monkeys was lower than that in the controls, and the vaccine prevented damage to the lungs. The prime–boost regimen induced humoral immune responses. Viral loads in the lungs were lower than in controls, but there was no reduction in viral shedding from the nose with either the prime-only or the prime–boost regimen. This suggests that AZD1222 may not prevent infection or transmission of SARS-CoV-2, but may reduce illness. The immune responses were not skewed towards a Th2-type and there was no suggestion of enhanced disease following vaccination. While a single dose induced antigen-specific antibody and T-cell responses, a booster immunization enhanced antibody responses, with a significant increase in SARS-CoV-2 neutralizing titres.263
The clinical studies are also based on the tests already conducted in the United Kingdom, Brazil and South Africa.
The pivotal safety, efficacy and immunogenicity data informing registration of the vaccine are derived from four ongoing studies:
- COV001, a phase 1/2 trial conducted in the United Kingdom;
- COV002, a phase 2/3 trial conducted in the United Kingdom:
- COV003, a phase 3 trial conducted in Brazil; and
- COV005, a phase 1/2 trial conducted in South Africa.264
The above is most probably what informed the decision of the WHO to support the Oxford/AstraZeneca without bothering to answer the salient questions that are being asked by the critics of the vaccine.
Meanwhile, it has, however, not gone unnoticed that it is precisely most of the developing countries that have imported the Oxford/AstraZeneca vaccine in large quantity. But it has also not gone unnoticed that some of these developing countries are former British colonies like Nigeria that hastily came to declare the vaccine is safe when it knows next to nothing about what has transpired with the vaccine. This can only be understood in the context of hangover of the debilitating colonial mentality, of which dependency syndrome is the major feature, and a powerful feature that has become weaponized against the developing countries in form of neuroweaponry or epistemological warfare conducted by British realpolitik or avant-garde diplomacy. Developing countries are victims of their own insufferable abject dependency within the framework of North-South divide.
This same abominable but burdensome peripheral or sub-urban dependency on the global metropolitan centres demonstrated by the developing countries was equally displayed or exhibited towards China (a now acknowledged modern new colonial master through its Belt and Road Initiative) that is only too willing to supply whatever vaccination is demanded of it by the industrially-impotent developing countries. China is not only still actively engaged in its famed or infamous mask diplomacy of the Covid-19 era; it has now added vaccine diplomacy as a way of cornering the industrial eunuchs that are the developing countries that are middle and low-income earners in global ranking.
It is amazing how colonial hangover express itself in solidarity with the former colonial master in supporting a product that one has not contributed anything to its research and development, trying to reap from where it has sown any seed, while ignoring or neglecting indigenous capacity to develop similar vaccine of its own.
Meanwhile, British Prime Minister Boris Johnson has defended the safety of the AstraZeneca coronavirus vaccine. “That vaccine is safe and works extremely well,” Johnson wrote in The Times newspaper. “It is being made in multiple places from India to the US, as well as Britain, and it is being used around the world,” he added.265
Canada is another country (a former colonial outpost of Britain) that defended the vaccine even when it is not known for any form of involvement with the production of the vaccine.
Canadian Prime Minister Justin Trudeau also weighed in favour of the vaccine. “Our health experts … collect data continuously and they assure us that all the vaccines offered in Canada are safe and effective, including those from AstraZeneca,” Reuters quoted Trudeau as saying. “We are obviously watching what is happening with a specific batch in Europe. We can reassure all Canadians that no AstraZeneca doses came from the same batch,” he added.266
It has not also gone unnoticed that it is precisely these developing countries that were probably the staunchest supporters of WHO when the latter was under immense pressure from the developed countries especially the United States under the former President Donald Trump. But here is the Catch-22: WHO strenuously prevented any form of indigenous herbal cure or hybrid vaccine from any developing country. The world has not forgotten the hostile treatment given to Madagascar by WHO. The world has not forgotten how WHO treated Taiwan. Thus WHO speaking in contextual respect of the developing countries as regard the need to take the AstraZeneca jab is understandable in turn in context of the support it as enjoyed from them. But in the final analysis, WHO is not in any way a friend of the developing countries as it claimed going by its double-standard.
However, interesting is the fact that the chummy relationship between the developing countries and WHO did not prevent the former from suffering the collateral damages of the coronavirus pandemic. Indeed, worst of all, WHO’s barefaced shenaniganism has gone a long way to reinforce the corrupt practices that were evidently noticeable in many developing countries and allow them to get away from public accountability. Nigeria is a notable example in this ignoble scenario.
Conclusion
Looking over the arch of time and space (from January 2020 to January 2021) in the making of the Oxford/AstraZeneca (AZD1222) vaccine and the fireball of controversy it has generated not only within the context of geopolitics of the European Union, but also the concerns it has generated all over the world, it is very obvious that the management of the vaccine was flawed from the very beginning from the boardroom of AstraZeneca.
This becomes more apparent when considered against the fact that other vaccines that were also in the making during the same period did not generate so much controversy (probably only with the exception of Russian Gamaleya Sputnik V vaccine) the way the Oxford/AstraZeneca vaccine has done. The blame for this unnecessary controversy lay squarely on the shoulders of the Management of AstraZeneca and the British Government especially in their denial of possible errors/mistakes, the business model adopted for the production and distribution of the vaccine, the very poor public relations management for the raised concerns on the public domain.
The world now live in an accelerated environment of informatized and digital age where an “innocent” mistake can be amplified and blown out of proportion ten thousand times over within a very brief period. The world now live with Internet, social media, digital and automated media that disperses information and disinformation very fast, crunches numbers and break them down into particles akin to nuclear accelerators for breaking down atoms into their constituent particles. Oxford/AstraZeneca did not take this phenomenal environment into consideration at all in its public relations management of the controversy that blew up on its face by its clumsy handling of the vaccine from the experimental laboratory to the street trials and testing and finally to the global market place.
At the core of the controversy was whether Oxford/AstraZeneca made a genuine mistake or error in the course of producing the vaccine especially during the trials and tests of the vaccine on volunteered patients that produced two different set of results in UK, Brazil and South Africa or it deliberately doctored, falsified, tampered with the results when the error was discovered.
It is indeed very hard or difficult to even consider the possibility that the University of Oxford team of scientists that kick-started the vaccine (such as Professor Teresa Lambe, Professor Sarah Gilbert, Professor Andrew Pollard, Professor Adrian Hill, Professor Katie Ewer and Professor Katherine Green and many more) renowned scientists who have built a career of scientific integrity up till this moment would ever be involved in the dirty act of tampering with results when they were not too favourable. It is simply too hard or difficult to believe they ever did such a thing.
Yet this is what a dispassionate analysis of processes and events in the course of this vaccine is suggesting. How this could have ever happened at all is another extremely hard or difficult thing to believe.
It is rather unfortunate to be forced to conclude that the analytical results of the two-dose regime for the vaccine test may have been doctored, falsified, modified, corrected, tampered with, etc (each word or phrase with its different connotative interpretations including civil or criminal liability) in order to rush and reach the global market place with the vaccine with the expected or anticipated accolades and celebration.
There is no doubt that there is a battle for supremacy among the Big Pharma companies in the break-neck race to produce a vaccine. Oxford/AstraZeneca vaccine was equally caught in this sort of “rat-race” alongside their cousins in America: Pfizer, Moderna, Jansen, Johnson&Johnson, etc. Unfortunately, in this “rat-race” or horse-race, Oxford/AstraZeneca vaccine got crashed, wounded or badly bruised and ended at the finishing lane in a hailstorm of controversy. British Government and World Health Organization could not help even when both threw their support behind the vaccine. They could not prevent the European countries from going for the throat of Oxford/AstraZeneca by rejecting its vaccine, denying it entrance into their countries for administration on their citizens. “Not fit for human consumption/vaccination here” is what seemed to have become the fate of the vaccine in the countries that rejected it.
It is a dangerous gamble, the result which was now what is being witnessed as the Oxford/AstraZeneca (AZD 1222) vaccine stumble from one roadblock to another, reeling from the negative publicity it has received and swimming against the tidal wave of expert and public opinions.
What became noticeable in the firefight is the adamant refusal of the top Management of AstraZeneca to accept the possibility of mistake being made inadvertently in the course of the vaccine production by the team of Oxford scientists. That obstinacy became a catalyst for emboldened public opposition and further inquisitiveness into what has been perceived or believed to have gone wrong along the line. From all pieces of evidences and connection of dots, the public believed that AstraZeneca is hiding something somewhere that it does not want to be publicly known.
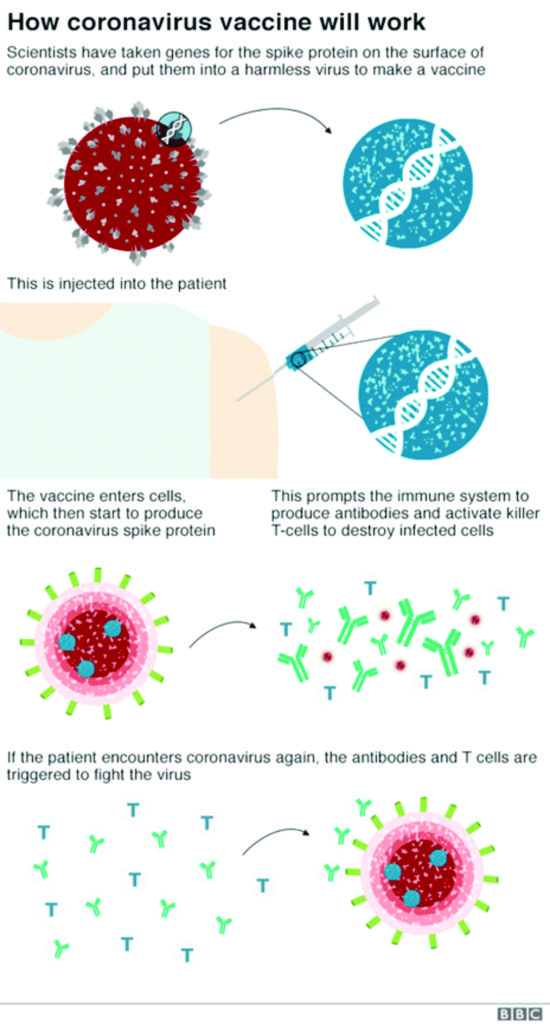
The EU countries that have rejected the vaccine ostensibly acted in their so-called “national interests” and nobody can reasonably blame them for that – even if hypocrisy is seen to be embedded in their peremptory actions. It is not a question of the number of patients that have died as a result of taking the jab from Oxford/AstraZeneca vaccine – which in the final tally is infinitesimal. But the fact that anybody could died at all and linked with the vaccine is the main issue, even if it is a lone individual. Science looks at a global picture while making provision for accident that could happen at any unpredictable point in time.
First, British Government cannot be said to have handled the crisis very well at all. Boris Johnson-led Government has not earned any public support over its management of the coronavirus pandemic throughout 2020. It would be recalled that Boris Johnson was actively engaged with Brexit celebration in January and February 2020 when coronavirus pandemic came calling. Boris Johnson most probably did not believe in the pandemic, in unison with former President Donald Trump of the United States, until the virus sent him to the hospital in April 2020. It was not until he came out of the hospital humbled while recovering from the coronavirus infection that he started running from pillar to pole trying to impose lockdown and other safety measures. By this time, it was already too late as the pandemic increase exponentially, spreading throughout the British Isles, turning the United Kingdom into one of the top-five worst-hit countries in the world. Britain has nothing to showcase to the world as a success story about the coronavirus pandemic.
By the time the Oxford/AstraZeneca vaccine came into the global market shelf in late 2020, coronavirus pandemic has claimed over 100,000 lives of British citizens. Even while vaccine was being administered to the beleaguered citizens, Brits are still dying in droves like flies while new variants have also emerged to the dismay of the British scientific community.
Second, when finally Oxford/AstraZeneca top management and the British Government became aware of the growing opposition and rejection of the vaccine by several of the EU members including Germany, France and Italy, (whether justified or not) they chose to confront them as if they were their former vassals, instead of embarking on shuttle diplomacy to douse the growing hostility and rejection of the vaccine. The very fact that three members of the G-7 countries of which Britain is also a member have decided to be at dagger-drawn with Britain over the vaccine by rejecting it should have alerted Whitehall and No 10 Downing Street that there is a proverbial fire raging on the mountain! That fire is, of course, not without a smoke. The fire was ignited in the Oxford laboratory which the British Government should have investigated instead of been seen to be siding with those who or what started the fire.
In this type of situation there must be no room for any iota of doubt about the efficacy of the vaccine. The philosophical principles of exact science still dominate even though it may sometimes be viewed from the lens of relativism. When and where is such a doubt, the Management must be seen coming out clean to admit whatever errors are found, allay the fears of the public that is ultimately the end-users and decider of the fate of the vaccine. It is the first law and principle. Oxford/AstraZeneca violated this law and principle by grandstanding the public, looking at them as ignorant lot, acting with impunity and damning the consequences of its action without regard to public sensitivity. It is not too much in this contextual circumstance to ask the Pascal Soriot-led leadership of AstraZeneca company to resign for its failure to handle this controversy very well, for its arrogance and impunity.
Oxford/AstraZeneca without doubt was confronted with the problem in its supply chain, i.e. the inability to meet demand especially from the European market which led to subcontracting the production of the vaccine to other companies outside Britain, a situation that also generated its own logistic problems that have collectively gone to complicate matter for Oxford/AstraZeneca. From this standpoint, it is clear that Oxford/AstraZeneca has bitten more than it can chew, gave false promises that it can deliver 40 million doses instead of 30 million doses in a quarter, thus going forward and backward. Oxford/AstraZeneca should have calibrated its production capacity very well in advance and limit itself to what it can provide within the framework of the competitive global environment of Covid-19 vaccine production. Naturally, the shortfalls will be taken over by other competitors.
The climax of it all is the corporate arrogance that breeds public insensitivity by Oxford/AstraZeneca. It is the same with Boris Johnson-led British Government.
The well-known voluble and caustic British Press is at its wits’ end, unable to convincing explain what has happened, indeed has little to offer in defense of this unfortunate incident. The British Press was caught at the wrong end of the rope. From the personal view of this writer, even as a journalist, based on the hundreds of news reports reviewed for this article, either cited in this article or not, I am of the view that the British Press was cornered like a rat in an embarrassing ambivalence over this incident. The British Press could not outrightly condemn the incident, could not condemn the sloppy British Government. But neither could it also come out to condemn the host of countries that have rejected the vaccine as that option would have been suicidal. It is not one or two that rejected the vaccine but more than a dozen which include members of the elitist G-7 countries, i.e. the giants of Europe (Germany, France and Italy). The British Press cannot take on these countries hoping to take them down with their well-known grammatical dexterity.
Even the British tabloids, the Altsatian attack dogs,were muffled. They could not bark or bare their canines. But if it to be any of the developing countries that were guilty of his cosmic malfeasance, one can imagine what would have happened to such an unfortunate country. The Alsatian or Labrador attack dogs would have come out as a cohort to tear apart and finish off such a hapless country.
They could not speak their high-falutin grammars, the types witnessed in the description and characterization of former President Donald Trump of the United States.
Meanwhile, there are those countries that have rushed to buy and import the Oxford/AstraZeneca vaccine in large quantities for their respective countries with or without minding the controversy that has come to engulf the vaccine. First, let it be recalled here that University of Oxford officialdom actually wanted the vaccine to be distributed on not-for-profit basis while AstraZeneca argued that that it could be subsidized for EU countries but sold to others on profit basis. The vaccine has now gone abroad to the developing countries on profit transactional basis. But that may not even the cause of concern over the behaviour of the developing countries that ostensibly has not outgrown their colonial legacy of mental slavery or skullduggery to the imperialist metropolitan centre in London. What baffles the well-informed circles is the haste with which the developing countries have imported the vaccine into their countries to be administered on their citizens without waiting for the controversy over the efficacy of the vaccine to be settled. It is a suspicious policy decision and move when considering the fact that a country like Nigeria, for instance, suffered far less infection and mortality rates than most of those countries that have rejected the vaccine.
The developing countries are victims of their limited knowledge and worldview about the coronavirus pandemic. There are people who believed that coronavirus itself is a biochemical weaponry contrived by China for reasons best known to it. There are now those who also view vaccines and not just Oxford/AstraZeneca vaccine alone, as an extension of this coronavirus biochemical weaponry. In other words, vaccines could be weaponized for political reasons, whether one is aware of it or not at all. The developing countries are victims of their abominable incapacity to manufacture their own vaccines but forced to rely on foreign sources. They are victims of their abject dependence on imported essential drugs and vaccines over the ages.
No one knows as at present how and where the controversy would end. Until then it could have been better to keep fingers crossed.