By Alexander Ekemenah, Chief Analyst, NEXTMONEY
Introduction
“It’s not hard to see that we’re in the middle of a once-in-several-generations economic crisis with a once-in-several-generations public health crisis. A crisis of deep human suffering is in plain sight and there’s no time to waste. We have to act and we have to act now”
– President-elect Joe Biden said on January 14, 2021
The battle against coronavirus pandemic (SARS-CoV-2) worldwide is still on because the pandemic is still raging furiously. The fallouts or the collateral damages of the pandemic are still far-flung though the degree differs from one country to another. As the saying goes: “it is not over until it is over”.
But both the pandemic and the battle against it has reached a critical turning point. The pandemic has reached a stage where it is now being met with stiff resistance from humanity as represented by several governments across the world. The pandemic has proven beyond all reasonable that it constitute a clear and present danger to humanity and it has to be confronted as a dangerous enemy. On the other hand, the battle against it has also reached its own critical stage with the ongoing global vaccination exercise as an interventionist instrument to combat the pandemic. How this interventionist instrument will work out in the final analysis is still uncertain as the ongoing vaccination exercise is yet to prove its efficacy beyond all reasonable doubt.
It is, however, a great philosophical irony of time that the world find itself with the coronavirus. From the very beginning, the more the pandemic continue to claim lives worldwide, so also are some people daily smiling to the banks with the proceeds from research grants, donations, government intervention funds, and more propitiously sales of vaccines worldwide that are now considered to be key to lowering the infection and mortality rates in the push to finally end the pandemic. The ongoing manufacturing of the vaccines (at mass production rate) comes at a specific cost, to be sold to governments at specific prices and to be administered to the end-users also at a specific cost, whether subsidized or not. There is, therefore, not only a cost-benefit framework, there is also the balance sheet consideration which we can only get to know very much later. In other words some people are making profit on the back of the misfortunes of all the millions of people affected and claimed by the pandemic. It is indeed a great irony of how misfortune of millions of people turns to fortune for few people!
Big Pharma conglomerates are now busy rushing to the global market place with various forms and brands of vaccines that were not available at the height of the raging pandemic last year. All of them claim that their vaccines are safe enough to be administered to the people. Now, dozens of various types of vaccines can now be ordered by governments for their respective individual countries and citizens battered by the coronavirus pandemic. But a quick glance at the Big Pharma conglomerates shows none of them to be either located in the middle-income or low-income earning countries. In other word, majority of the countries in the world is still yoked and made dependent on the high-income earning countries – at least a few top-cloistered ones – that are able to manufacture the vaccines.
The inequality in global distribution of ownership, production and power of essential drugs, in this case Covid-19 vaccines, have now become glaring. The monopoly of ownership and production of these essential drugs and vaccines by the rich North as against the poor South has become indisputable as a matter of fact. The coronavirus pandemic started from the second most powerful economy in the world, China, with all its inescapable moral and legal blameworthiness. The same China is now also a major competitor in the global market of vaccine production and selling to the victims. It is a situation of starting the global pandemic panic and then selling the balm to calm down the global overwrought nerves!
There is also evident ferocious competition among the Big Pharma manufacturers of vaccines. There is covert or overt battle for supremacy among the vaccine makers. Which vaccine is the most effective? As at the time of publishing this article, a major vaccine has ran into troubled waters for causing blood clot that leads directly to death. Who will sell the most vaccines especially among the middle- and lower-income earning countries who are now being spinned on the finger-tips of the Big Pharma vaccine makers and their corresponding Governments? Who will win between the vaccine nationalists and the internationalists?
The desperation of countries of high-income, middle-income especially the low-income earning countries that did not hitherto have the foresight to invest in their health sectors and with particular reference to production and storage of vaccines not to be left out of the global rush for the vaccines reached its peak when it was announced by The Philippines Government to send thousands of their nurses to migrate to the United Kingdom and Germany in exchange for vaccines.1 “Health workers is most severe in poorer countries. For example, the first global State of Nursing report issued by the World Health Organization in 2020 shows that 83% of the 5.9 more nurses needed across the world are in low- and lower middle-income countries, while over 80% of the world’s nurses are found in countries that account for half of the world’s population.”2
“This unilateral offer by the Philippine government, which is unacceptable under any condition, does, however, reflect the desperation which developing countries like the Philippines are facing, given the current global regime of inequitable access to vaccines”3
Indeed, the situation in some countries can only be spoken of with trepidation or nausea given the historic proportion of corruption and incompetence within their Governments. Nigeria is one of such countries where some government officials and politicians have earlier sneaked out of the country to travel overseas especially to Middle East, European and North America to get vaccinated even before the arrival of the first consignment of vaccines into the country. They have videos and pictures posted online and on social media, flaunting their connections and taunting the masses for their abject poverty that prevents them from travelling to get vaccinated. Vaccination is now classed as a high status symbol!
The Punch newspaper, one of the leading and most influential Nigerian newspapers, in its flag headline, reported that Nigeria is going to spend N10.6 billion to transport and distribute 3.9 million doses of the vaccines already imported into the country.4 It is heart-breaking to know and read of such developments. The questions that necessarily arise here could be legion. Where are the special vehicles that will transport the vaccines across the length and breadth of the country? Are the “cold room” facilities already available and prepared in advance to receive the vaccines, store them till the time they would be administered to the members of the public. Is uninterrupted electricity supply assured and guaranteed in all places where the vaccines are going to be stored before their administration? Has Government even considered the possibility of road accidents on our bad roads thereby getting the vehicles crashed and destruction of the vaccines? Has Government considered the problem of insecurity of lives and properties in the course of transporting the vaccines across the country in context of the level of banditry, kidnapping, armed robbery, insurgency and terrorism and the possibility of the transporting vehicles being waylaid, hijacked, diverted or stolen by armed gangs and other malevolent groups? Finally, why is Nigeria running from pillar to pole to secure the vaccines against the background of low infection and mortality rates in Nigeria compared to other worse-hit countries?
As of February 22, 2021, the United States had recorded over 28 million cases of COVID-19. The country had also reported a total number of almost 499 thousand deaths from the disease.5 The cumulative number of cases worldwide exceeded 100 million in late January 2021. The United States marked its own heartbreaking milestone a month later when the death toll passed 500,000. Demand for test kits has at times exceeded production levels, but countries must continue to test citizens to detect any spikes in cases effectively. The U.S. has performed most tests worldwide as of February 22, 2021.6
Widespread testing will also help to detect the hundreds of thousands of people who might be asymptomatic – showing few or no symptoms of the illness. These carriers are unwittingly transmitting the virus to others, and the threat of silent transmission is one reason why mass lockdowns have been imposed around the world. However, as asymptomatic carriers produce no symptoms, they may have developed some natural immunity to the illness. Viruses are not as easily spread in communities with high rates of immunity, which helps to protect more vulnerable groups of people. When an infection rate is less than one, a community has achieved herd immunity.7
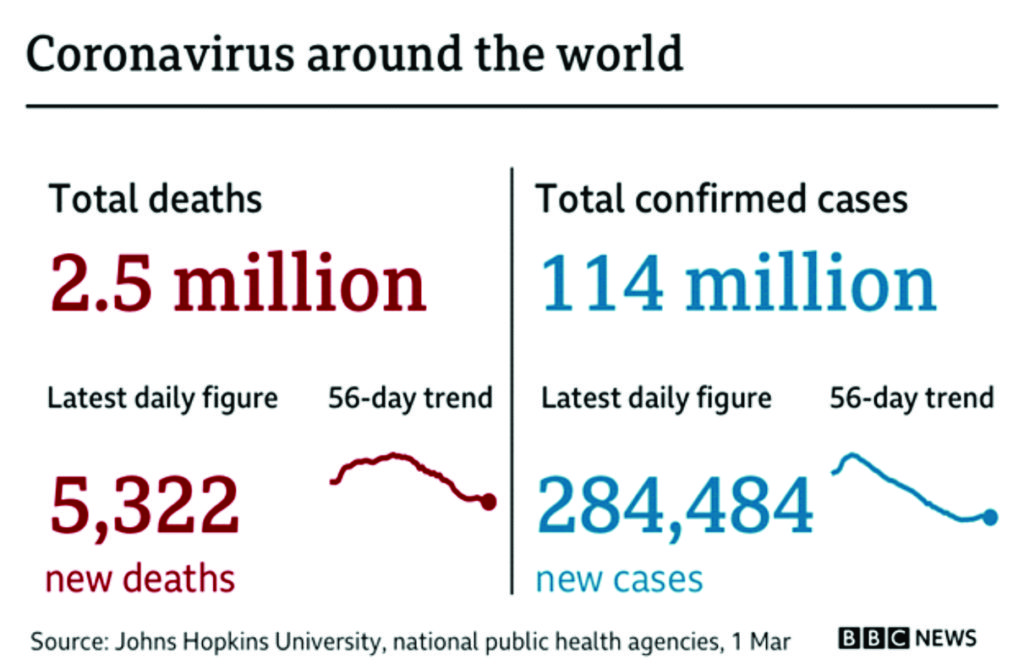
As at March 3, 2021, coronavirus cases worldwide stand at 115,418,203 while deaths stand at 2,562,863 with 91,209,445 recoveries.
BBC News presents a frightful global overview of the pandemic. Daily cases had been falling in most European countries earlier this month [February 2021], but several are now seeing a rise in infections. France, Italy, Russia and the Czech Republic have seen the highest numbers in recent weeks. Lockdown measures were tightened in many of the worst-affected countries over the winter but some restrictions are now being lifted.8
The US has recorded nearly 30 million cases and more than 510,000 deaths, the highest figures in the world. Daily cases were at record levels in early January but they have fallen substantially in the last few weeks. Canada, which has a far lower death rate than the US, also experienced a winter surge but daily cases have also been falling recently.9
Asia was the centre of the initial outbreak that spread from China in early 2020, but the number of cases and deaths there has been lower than in Europe and North America. The region saw a large rise in the number of cases last autumn [2020], driven by a surge in infections in India, one of the most densely populated countries in the world. India has seen more than 11 million confirmed cases, the second-highest in the world after the US, but the number of daily infections has fallen in recent months.10
Several countries in the Middle East have had deadly coronavirus outbreaks over the past 12 months, with Iran and Israel having seen the highest numbers. Cases are now falling in Israel but several countries in the region are currently seeing renewed outbreaks, including Iran and Iraq. Israel’s efforts have been helped by its vaccination programme, with about eight million doses administered.11
Africa has recorded nearly four million cases and more than 100,000 deaths – but the true extent of the pandemic in many African countries is not known as testing rates are low. South Africa, with about 1.5 million cases, is the worst affected country on the continent, according to official figures. Morocco, Egypt, Ethiopia, Tunisia, Libya, Algeria, Nigeria and Kenya have also recorded more than 100,000 cases. Ivory Coast became the first country to roll out vaccines offered by UN-backed Covax programme, starting with healthcare workers on Monday. Ivorian authorities will initially receive half a million doses of the AstraZeneca Oxford vaccine. Ghana and Nigeria are also due to start their inoculations this week [i.e. early March]12
In Latin America, there has been particular concern about a variant of the virus that has been spreading rapidly in Brazil. The country has more than 10 million confirmed cases and 250,000 deaths – the world’s second highest death toll. It is currently in the middle of a surge in infections. Argentina, Colombia and Mexico have all recorded more than two million cases while Peru has seen more than one million.13
Australia and New Zealand have been praised for their response to the pandemic, with both countries having seen comparatively few deaths. In a sign of how effective their lockdown measures have been, both New Zealand and Australia currently have a lower average number of cases than French Polynesia, a sprawling network of islands in the Pacific Ocean. Other islands in the region have tried to remain free of coronavirus, but most have seen at least a few cases. Papua New Guinea is currently the worst-affected country in the region after seeing a recent spike in cases.14
The declining infection and mortality rates in the United States where they are the highest in the world were captured and carefully interrogated by Alexander Ekemenah (2021)15
One of the key factors argued to be responsible for the decline is the ongoing vaccinations of Americans which have now reached a commendable proportion (the highest in the world so far, even surpassing China) within a very short time frame – which in turn shows the seriousness of commitment invested in the vaccination endeavor by Joe Biden-led Administration as against the visible lackadaisical or cavalier attitude hitherto displayed by the previous Trump Administration. While vaccination seems to be a valid argument for the declining infection and mortality rates, it is still a highly debatable point essentially because there are no yet preponderant evidences that Covid-19 vaccination would push back and/or halt the spread of the pandemic. The interplay of other factors is fundamental to the final pushback and halting the pandemic.
However, since mid-January 2021, it has become noticeable that the hitherto raging pandemic is now seen to be slowly declining with particular reference to the United States where more than 500,000 have lost their lives to the pandemic. Bloomberg notes that the pandemic has lost momentum globally with total infections growing at the slowest pace since October. It notes further that even as it marks the grim milestone of 500,000 lives lost, the U.S. is potentially at a turning point in its outbreak, the worst in the world.
Bloomberg gives a simulacra of the slowly de-accelerating pandemic across the globle in its Covid Resilience Ranking conducted by Jinshan Hong, Rachel Chang and Kevin Varley (2021) The Covid Resilience Ranking is a snapshot of how the coronavirus pandemic is playing out in 53 major economies right now. By grading their vaccine orders and the progress of distribution, we also provide a window into how these economies’ fortunes may shift in the future.16 It’s not a final verdict, nor could it ever be with imperfections in virus data and the fast pace of this crisis, which has seen subsequent waves confound places that handled things well in initial waves. Circumstance and pure luck also play a role, but are hard to quantify.17
Vaccine rollout and access is proving to be a decisive factor in 2021, with challenges from logistics and storage to vaccine hesitancy. Still, having endured over a year of fighting Covid-19, governments and populations now have a better understanding of the elusive pathogen, how best to curb its spread and mitigate the damage it inflicts.18
Nearly a year since Covid-19 was declared a pandemic, the U.S. and parts of Europe are emerging from the darkest chapters yet of their outbreaks and climbing up Bloomberg’s Covid Resilience Ranking, a measure of the best places to be in the coronavirus era.19
While headlines have been dominated by the rush to vaccinate, these countries’ gains have largely stemmed from containment measures like mask-wearing and staying home. The U.S., led by the new administration of President Joe Biden, is also poised for a faster-than-expected economic rebound. Its rank leapt eight spots in February to 27th.20
Globally, the pandemic has lost momentum with total infections growing at the slowest pace since October. But major western economies are gaining ground faster than developing nations, fueling a rich-poor disparity in the Ranking that’s likely to persist in 2021 given the domination of vaccine supply by wealthy governments.21
Snuffing out or containing Covid early continues to pay off in quality of life for places like New Zealand, Taiwan and Australia, which have been in the Ranking’s top 10 since its first edition in November. A major gap remains between these top performers and the rest of the world, despite their vaccination drives trailing the U.S., U.K. and Europe.22
Wealthy economies previously in the bottom 10, like France, Belgium and Italy, have climbed since November, pushing down countries like South Africa and Indonesia. The Ranking’s bottom third is now populated by developing economies in Latin America and Africa.23
India, which to the puzzlement of scientists seems to have evaded the worst of Covid-19 despite its huge outbreak, moved up two spots to 16th as fatalities continued to fall.24
The domination of vaccine supply by richer countries—which the World Health Organization Director-General Tedros Adhanom Ghebreyesus called a “catastrophic moral failure – will likely prevent poorer nations from moving up the ranks in the coming months. Mexico remains at No. 53, the last of the ranked economies.25
Israel—the global leader in inoculations—is providing real-world proof that the experimental mRNA vaccines work not just to prevent deaths but to also slow transmission. But its rank in February inched up only one place to 14th with the rapid vaccine rollout yet to fully quell a wave of the highly infectious U.K. variant across the country, reflecting the danger that mutations continue to pose.26
The Ranking scores economies of more than $200 billion on 11 core metrics: from growth in virus cases and the overall mortality rate to testing capabilities and vaccines. The capacity of the local health-care system, the impact of virus-related restrictions like lockdowns on the economy, and freedom of movement are also taken into account.27
Even as it marks the grim milestone of 500,000 lives lost, the U.S. is potentially at a turning point in its outbreak, the worst in the world. Cases have fallen by half from a month ago, and deaths are also on a downward trajectory. Mask-wearing, which became politically polarizing in parts of the U.S., is now at an all-time high of 77%, according to the Institute for Health Metrics and Evaluation.28
Half of Israel’s population of 9 million have had at least one dose of a Covid shot, providing the world with a glimpse of what the vaccinated future has in store. Life for those who have been inoculated is normalizing fast, with the “Green Pass”—issued to people who complete the vaccine regimen or recover from infection—allowing them to use gyms, hotels and swimming pools. The country signed agreements with Greece and Cyprus to allow vaccinated citizens to visit unimpeded once travel opens back up. Data gleaned in Israel’s rollout indicates the Pfizer Inc.-BioNTech SE shot prevents almost all Covid deaths, and also stops the majority of inoculated people from contracting the virus.29
Still, it’ll take more time for its outbreak, fueled by the U.K. variant to one of the worst infections per capita rate among the ranked economies, to be contained. There’s also been an uptick in seious cases among younger people, who are at the back of the vaccine line.30 Inoculation will be “a critical factor in reducing the death toll in the next four months, especially if vaccination is scaled up before the new variants spread,” said Christopher Murray, director of the Institute for Health Metrics and Evaluation at the University of Washington in Seattle.31
Top-ranking places in the Asia-Pacific region have generally moved slower on vaccines, which officials defend as due to caution. With their outbreaks largely contained and deaths low, these economies can arguably afford to wait and learn from how the unprecedented vaccine drives play out elsewhere.32 But that go-slow approach also runs the risk of disadvantaging them economically and their edge in the Ranking could be blunted as vaccination starts to translate into normalization of life—and the resumption of international travel—in other places.33
Forcing Vaccination Down the Throat of the Public
The propaganda to attract the sympathy of the public to the vaccination roll-out around the world is massive and is increasing exponentially, to say the least. Every country is mounting propaganda to attract public support for the vaccination exercise. For instance, according to The Our World in Data COVID vaccination data website: To bring this pandemic to an end, a large share of the world needs to be immune to the virus. The safest way to achieve this is with a vaccine. Vaccines are a technology that humanity has often relied on in the past to bring down the death toll of infectious diseases.34 Within less than 12 months after the beginning of the COVID-19 pandemic, several research teams rose to the challenge and developed vaccines that protect from SARS-CoV-2, the virus that causes COVID-19.35 Now the challenge is to make these vaccines available to people around the world. It will be key that people in all countries — not just in rich countries — receive the required protection.36
COVID-19 vaccines are effective at protecting you from getting sick. Based on what we know about COVID-19 vaccines, people who have been fully vaccinated can start to do some things that they had stopped doing because of the pandemic.37
We’re still learning how vaccines will affect the spread of COVID-19. After you’ve been fully vaccinated against COVID-19, you should keep taking precautions in public places like wearing a mask, staying 6 feet apart from others, and avoiding crowds and poorly ventilated spaces until we know more.38
People are considered fully vaccinated:
- 2 weeks after their second dose in a 2-dose series, like the Pfizer or Moderna vaccines, or
- 2 weeks after a single-dose vaccine, like Johnson & Johnson’s Janssen vaccine
If it has been less than 2 weeks since your shot, or if you still need to get your second dose, you are NOT fully protected. Keep taking all prevention until you are fully vaccinated.
If you’ve been fully vaccinated:
- You can gather indoors with fully vaccinated people without wearing a mask.
- You can gather indoors with unvaccinated people from one other household (for example, visiting with relatives who all live together) without masks, unless any of those people or anyone they live with has an increased risk for severe illness from COVD-19.
- If you’ve been around someone who has COVID-19, you do not need to stay away from others or get tested unless you have symptoms.
- However, if you live in a group setting (like a correctional or detention facility or group home) and are around someone who has COVID-19, you should still stay away from others for 14 days and get tested, even if you don’t have symptoms.
For now, if you’ve been fully vaccinated:
- You should still take steps to protect yourself and others in many situations, like wearing a mask, staying at least 6 feet apart from others, and avoiding crowds and poorly ventilated spaces. Take these precautions whenever you are:
- In public
- Gathering with unvaccinated people from more than one other household
- Visiting with an unvaccinated person who is at increased risk of severe illness or death from COVID-19 or who lives with a person at increased risk
- You should still avoid medium or large-sized gatherings.
- You should still delay domestic and international travel. If you do travel, you’ll still need to follow CDC requirements and recommendations.
- You should still watch out for symptoms of COVID-19, especially if you’ve been around someone who is sick. If you have symptoms of COVID-19, you should get tested and stay home and away from others.
- You will still need to follow guidance at your workplace.
- We know that COVID-19 vaccines are effective at preventing COVID-19 disease, especially severe illness and death.
- We’re still learning how effective the vaccines are against variants of the virus that causes COVID-19. Early data show the vaccines may work against some variants but could be less effective against others.
- We know that other prevention steps help stop the spread of COVID-19, and that these steps are still important, even as vaccines are being distributed.
- We’re still learning how well COVID-19 vaccines keep people from spreading the disease.
- Early data show that the vaccines may help keep people from spreading COVID-19, but we are learning more as more people get vaccinated.
- We’re still learning how long COVID-19 vaccines can protect people.
- As we know more, CDC will continue to update our recommendations for both vaccinated and unvaccinated people.
Until we know more about those questions, everyone — even people who’ve had their vaccines — should continue taking basic prevention steps when recommended.39
What has become evident is that there has been a noticeable shift of emphasis from the safety measures consisting of wearing of face masks, keeping social distances, washing of hands regularly and sanitizing of hands to vaccination. All countries have lifted their lockdowns and stay-at-home orders in view of their devastating impact on the economic livelihoods of people worldwide. Vaccination has now taken the centre stage as most governments are not only encouraging through moral suasions but seemingly forcing through coercion of citizens to take the vaccine and show evidence of having taken the vaccine through certificates, etc. All the previous personal freedom or right to choose as relate to wearing of face masks, etc, can now be seen to being abrogated/abolished in favour of forcing citizens to take the vaccines. The citizens can hardly claim any fundamental rights to exercise his freedom of choice to take the vaccine or not.
Meanwhile, there are millions of those who have morbid fear of taking the jab for variety of reasons, whether justified or not. One of the reasons is the allegation that the vaccination is meant to reduce population of certain groups of people, by killing them slowly over time and space; or worst of all to make them infertile by destroying their reproductive organs, etc. Governments have not been able to allay the fears of these “refusniks”!
But the exercise raises serious philosophical and ethical questions from local to global perspectives. To what extent can Governments force their citizens to undergo vaccination? Where is the right of conscientious objections from many standpoints: political, religious, moral and ethical or philosophical? From all indications, it seems government would not be happy if a citizen refuses to take the jab. So to what extent can government go to inflict punishment on such an individual or group of individuals? Can government criminalize refusal to take the jab? Would government be justified in doing so, in view of the massive failure of the same government in stemming the tidal wave of the pandemic in overwhelming the citizens in the first instance? Is forceful or coerced vaccination not an illegal invasion of the privacy of the citizens even under the context of public health safety? Where is the dividing line between privacy and concerns about public health safety? How do government compensate victims who died from taking any of the jab? If Western democracies are arm-twisting their citizens to take the jab, do they then have the right, apriori, to criticize authoritarian regimes for violating the rights of their citizens in respect of this pandemic as we have hitherto been inundated in the media? About 46% of Republicans in the US have been reported saying they will not take the jab. What does the Federal Government/Biden Administration intend to do about that?
These questions can not be answered here. Attempt will be made to answer them in another write-up.
Vaccines’ Manufacturers Smiling to the Banks
One of the dynamic features of the pandemic is the push for cure through vaccines as the pandemic rages worldwide. Other features include the various measures taken by different governments worldwide to halt the spread of pandemic. While few governments succeeded early enough in halting the pandemic in their countries (mostly in the South East Asia whom have been collectively referred to as the Mekong-Pacific Knights) most other governments especially in Europe and Americas failed calamitously in this national emergency task of halting the pandemic. The Mekong-Pacific Knights have shown no known desperation for vaccination because they have since conquered the pandemic. It is, rather, the countries that allowed themselves to be so ravaged by the pandemic as to make them lying hopelessly prostrate that have shown high desperation at getting vaccination!
Interestingly, it is not countries such as the Mekong-Pacific Knights that have succeeded in halting the pandemic in their respective countries (with the exception of Japan) that are at the forefront of the discovery of the vaccines. Some of them are high income earners such as Taiwan, Australia and New Zealand. Rather, it is unexpectedly the very countries in Europe and Americas that have failed so calamitously that are at the forefront of production of Covid-19 vaccines for the world market today. Necessity has been proven to be mother of invention. The coronavirus pandemic unexpectedly came out of the blue sky to hit them full in the face with the consequence of desperately searching for vaccine cure. While the pandemic came as utterly strategic surprise to them, it also turned to become a goldmine for profiteering at the expense of the victims and a world held hostage by the pandemic. Global capitalism has shown its utter rottenness!
The global vaccine market can be claimed to have reached a watershed with the outbreak of the coronavirus pandemic. Even though coronavirus pandemic has not killed people as the Spanish Flu of 1918 did, it is considered one of the deadliest the world has ever encountered. There is international urgency to halt it through individual and collective efforts by governments and multilateral bodies through collaboration, etc. However, apart from governments and multilateral bodies, Big Pharma multinational corporations involved in research and development of vaccines have now taken the international lead in discovery and production of necessary vaccines against the coronavirus pandemic.
Thus suddenly the global vaccine market can be seen to be undergoing revaluation in terms of profit and loss with outbreak of the coronavirus pandemic. It is an internal evolution caused by objective external factor which is the outbreak of the pandemic in late 2019. As the baseline for this analysis we can look at the global vaccine market from 2015 to 2019 to see the character of this evolution of global market value interconnected with the outbreak of the coronavirus pandemic.
Another characteristic feature of global vaccine market is what has been tagged “vaccine nationalism” in which case the countries where the vaccine manufacturers are domiciled displayed strong nationalism as regard the vaccine brands that came from their countries. None naturally came from the middle-income and low-income earning countries but are made dependent on the willingness of the vaccine manufacturers to supply their needs both on moral/health and profit grounds but definitely not on legal ground. Russia, China and India have shown to maintain their respective strong national identities with the vaccines produced in their countries. However, European and American (including Canada and Japan) commanded more international clout and strong brand status.
Even though Russia was the first to claim to have discovered Covid-19 vaccine with its Gamaleya Sputnik V brand, as far back as August 2020, it would soon be outstripped by the Western European and American brands such as PfitzerBioNTech, Moderna, AstraZenecca, Johnson&Johnson, Janssen, Covax, with their various degrees of effectiveness, etc. Of course, in the ongoing vaccination exercise in the United States and the United Kingdom, no vaccine was ever reportedly ordered from the Russians, Chinese or Indians. Rather, it has relied exclusively so far on vaccines produced in countries of their sphere of geopolitical influence – since it has been articulated that reliance on China, for instance, for their bulk of medical supplies have now become a grave threat to their national security interests as they are becoming captive to foreign domination, in this case, by China.
But as the pandemic has started slowing down and as vaccination goes full blast in both the United States and the rest of the world, medical experts and watchers of the pandemic are visibly worried about the emergence of new variants of the virus showing clearly what the world is dealing with here is a kind of hydra-headed monster that is yet is yet to be fully understood in its insidiousness as well as grave threat to lives and economies. This is another aspect of the pandemic that has baffled the scientific and medical officialdom – and an issue to look set to throw spanners into the work done and achievements already made. Where really these new variants come from?
In the United States, experts are warning states and jurisdictions to keep their mitigation measures in place in the face of new variants.40
Quoting the Centers for Disease Control and Prevention Director Rochelle Walensky the experts are of the view that it isn’t time to relax mitigation strategies yet. “We are continuing to watch these data closely, and although hospital admissions and cases are consistently dropping, I’m asking everyone to please keep your guard up. The continued proliferation of variants remains of great concern and is a threat that could reverse the recent positive trends we are seeing,” Walensky said at a press conference. The CDC director said she would “discourage” any relaxing of mitigation measures. She said the agency has documented nearly 700 cases of the variant first identified in the U.K. in the U.S., six cases of the variant detected in South Africa and three cases of a variant first found in travelers from Brazil.41
The global vaccine market value has been on the increase in the last decade or so.
The global vaccine market was valued at over USD 32.5 billion in 2015 and is expected to grow at a CAGR of 10.3% over the forecast period. The rising demand for better healthcare infrastructure and high awareness levels of the benefits of immunization are the major factors boosting the market growth.42 Governments across the globe are striving to ensure that every stratum of the society, irrespective of social and economic status is granted access to immunization. In May 2012, the WHO launched the ‘Global Vaccine Action Plan (GVAP)’ that was authorized by 194 member states of the World Health Assembly.43
This plan aims to strengthen routine immunization in order to check the transmission of communicable diseases through intermittent assessments to evaluate the achieved progress, measured in terms of national, vaccination coverage target goals that have been effectively met.44
The involvement of government and non-government organizations gaining prominence in this field is expected to provide the vertical with an impactful boost. The UNICEF and the WHO have published guidelines on developing a national immunization plan, the comprehensive Multi-Year Plan (cMYP) for all nations across the globe, which intends to ensure equitable access to vaccination facilities for all individuals and increase stakeholder participation in attaining vaccination coverage targets through the designing and implementation of feasible financial strategies to assess current and future program costs that increase the accountability of the respective participants.Worldwide immunization coverage, 2014.45
Various types of vaccines include the inactivated, attenuated, toxoid, subunit, conjugate, recombinant vector, and DNA vaccines. The inactivated vaccines segment is expected to dominate the market owing to the benefits associated with it, including a long shelf-life and high stability. The DNA vaccine is expected to witness the fastest growth over the forecast period due mainly to the high specificity and the reduced risk of integration into the genome as compared to other traditional vaccines.46
Moreover, the development of new and improved vaccinations for several diseases is a major factor projected to propel growth. The presence of several pipeline drugs is expected to lead to the growth of the Vaccine Market.47
Based on applications, the market is segmented into infectious diseases, cancer, allergy. Among these applications, the infectious diseases segment holds the largest share owing to the growing immunization concerns against a number of predominant infectious diseases, which are the major causes of mortality and morbidity, globally.48
The rising incidences of severe diseases, such as cholera, typhoid, hepatitis, measles, chickenpox, and many others have resulted in the sizeable growth of the global vaccines vertical due to the steady-paced increase in the demand for immunization against these diseases. However, several molecular entities are under extensive research and development stages, which are targeting immunization against fatal diseases, such as AIDS, Ebola, Hepatitis C, Leishmaniasis disease, Chagas disease, paratyphoid fever, and norovirus.49
North America is the most developed region and is expected to generate an estimated revenue of over USD 27 billion by 2024. The rising government support for vaccine development and the numerous company investments deployed for research and development are the key drivers accentuating the market growth in this region. Moreover, the established healthcare infrastructure and facilities present in this region are crucial factors supporting the emergence of this vertical.50 The Asia Pacific region is expected to witness the fastest growth over the forecast period, attributed by the growing target population base with high-unmet clinical needs. Furthermore, the increasing disposable income level and the rising awareness regarding the merits of vaccination in emerging economies of the Asia Pacific region is further expected to foster market growth.51
Some key players of this Vaccine Market include Merck &Co., Inc., Emergent BioSolutions, Inc., Johnson and Johnson, Sanofi Pasteur, Inc., Pfizer, Inc., Novartis AG, CSL Ltd., and GlaxoSmithKline Plc. Other prominent players include Abbott Laboratories, Inc., AstraZeneca Plc, Janssen Pharmaceuticals, Inc., Takeda Pharmaceuticals Company Ltd., and Valeant Pharmaceuticals International, Inc.52 Most of the companies are focusing on business expansion to attain a higher revenue share through the adoption of strategies, such as mergers and acquisitions and new product development. For instance, in September 2015, GlaxoSmithKline Plc completed the acquisition of the vaccine business division of Novartis and received approval for two new pediatric vaccines, Boostrix and Infanrixin India.53
The above analysis was made before the outbreak of the coronavirus pandemic. However, the pandemic has now brought new value to the global vaccine market.
According to the latest report by IMARC Group, titled “Vaccine Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2020-2025,” the global vaccine market size reached US$ 37 Billion in 2019.54
One of the leading trends witnessed in the global vaccines market is the surging cases of the coronavirus disease (COVID-19), which has, till date, resulted in the loss of around half a million lives around the world. As a result, several pharmaceutical companies are currently engaged in extensive research and development (R&D) activities to introduce a novel vaccine against the disease. Apart from this, the World Health Organization (WHO) is undertaking the initiative of increasing awareness about immunization through the Global Vaccine Action Plan (GVAP) and Global Immunization Vision and Strategy (GIVS). Moreover, the high prevalence of infectious diseases and R&D activities pertaining to the immunization of several fatal diseases, such as Acquired Immunodeficiency Syndrome (AIDS), Ebola, Hepatitis C and paratyphoid fever, are also propelling the market growth. Furthermore, the growing government support for vaccine development and the increasing involvement of global organizations in the development of adequate vaccination facilities in endemic regions is anticipated to positively influence the sales of vaccines in the upcoming years. Looking forward, the market value is projected to reach US$ 57 Billion by 2025, expanding at a CAGR of 7.40% during the forecast period (2020-2025).55
The competitive landscape include, apart from those already mentioned above, Abbott Laboratories, Astellas Pharma Inc., AstraZeneca Plc, Bharat Biotech International Limited, Bavarian Nordic A/S, CSL Limited, Daiichi Sankyo Company, Limited, Emergent BioSolutions Inc., GlaxoSmithKline Plc, Inovio Pharmaceuticals Inc., Johnson & Johnson, Inc., Merck & Co. Inc., Mitsubishi Tanabe Pharma Corporation (Mitsubishi Chemical Holdings Corporation), Novavax Inc., Panacea Biotec Ltd., Pfizer Inc., Sanofi Pasteur SA (Sanofi SA), Serum Institute of India Pvt. Ltd. and Takeda Pharmaceutical Company Limited.56
The global vaccines market was [further] valued at about $29.64 billion in 2018 and is expected to grow to $43.79 billion at a CAGR of 10.3% through 2022.57
Rising awareness on immunization and vaccination benefits in emerging markets is consistently driving the global vaccines market growth. World Health Organization (WHO) is taking initiatives to increase awareness of immunization through global vaccine action plan (GVAP) and global Immunization vision and strategy (GIVS).58
They aim to strengthen routine immunization, control morbidity and mortality from vaccine preventable diseases and help countries to immunize more people with a greater range of vaccines. The Organization accomplishes this work through its biological programme, the WHO Collaborating Centers, and the WHO Expert Committee on Biological Standardization (ECBS).59
In 2017, according to WHO statistics, 85% of the total infants across the globe, (116.2 million) received 3 doses of diphtheria-tetanus-pertussis vaccine in order to increase immunization and protect them against infectious diseases that can cause serious illness and disability. Furthermore, 123 countries reached 90% coverage of DTP3 vaccine in 2017.60
The vaccines market growth is limited due to severe shortage of skilled healthcare professionals for developing biologics drugs which requires specialized skillsets. These skillsets are limited to some research organizations and medical equipment companies in the USA and Europe, this is expected to be a major restraint on the market. As of 2018, 40% of the biopharma industry was facing difficulties in hiring for process development staff due to shortage of talent. This shortage also led to rise in competition and salaries for the limited talent pool available for biologics talent, thereby further limiting growth of the market.61
The vaccines market has been witnessing multiple strategic initiatives and mergers and acquisitions in the recent years. Top companies in the market are strategically acquiring start-ups and mid-sized companies to broaden products and services. For instance, in February 2019, Bharat Biotech acquired Chiron Behring Vaccines, a clinical biotechnology company, one of the leading manufacturers of rabies vaccines across the globe. Similarly, in 2017, Takeda Pharmaceutical acquired ARIAD Pharmaceuticals for approximately $5.2 billion, and Sanofi acquired Protein Sciences for $650 million.62
Companies in the industry are increasingly realigning their portfolios and pursuing profitable inorganic growth opportunities. Additionally, M&A interest is also being fueled by stronger corporate balance sheets, liquid debt markets, and continued favorable interest rates globally.63
The Food and Drug Administration (FDA) is the National Regulatory Authority (NRA) in the United States responsible for assuring quality, safety, and effectiveness of all vaccines for human use. Center for Biologics Evaluation and Research (CBER) within the US FDA is responsible for regulating vaccines market. Governments across the world are encouraging the research and development of biologics in order to develop more targeted therapies and vaccines for various diseases.64
In May 2014, the US’s FDA announced a fast-track initiative to review its drugs and biologics policy to speed the availability of therapies to patients with serious conditions, orphan drugs for rare disease, while preserving the safety and efficacy standards. In 2016, FDA also removed a rule (Section 610.21 of the FDA code) which specified minimal potency limits for certain antibodies and antigens. In addition, FDA is also updating regulations (Section 610.53 of FDA code) regarding storage periods and storage conditions for biologics.65
In October 2018, Emergent BioSolutions, a multinational specialty biopharmaceutical company, acquired PaxVax for $270 million. This acquisition would strengthen and expand Emergent BioSolution’s product portfolio with PaxVax’s typhoid vaccine Vivotif, cholera vaccine Vaxchora and additional clinical-stage vaccine used in the prevention of typhoid fever, cholera, chikungunya and other emerging infectious diseases. PaxVax is a company focused on developing, manufacturing, and commercializing specialty vaccines that protect against existing and emerging infectious diseases. PaxVax was founded in 2006 and is headquartered in Cayman Islands, a British Overseas Territory.66
The global vaccines market is projected to reach USD 58.4 billion by 2024 from USD 41.7 billion in 2019, at a CAGR of 7.0% during the forecast period. The growth of this market is majorly attributed to the high prevalence of infectious diseases, increasing company initiatives to enhance vaccine R&D, growing government support for vaccine development, and the rising focus on immunization. However, the huge capital investments required for developing vaccines may restrain market growth.67
North America accounted for the largest share of the vaccines market in 2018. The large share of North America in the global vaccines market is attributed to the high prevalence of infectious diseases and increasing investments by government and non-government organizations for vaccine development. The market in Asia, on the other hand, is projected to register the highest growth during the forecast period. Factors such as the increasing healthcare expenditure and increasing disposable income, government initiatives, and the presence of a large patient population are driving the growth of the vaccines market in Asia.68
Compared to the pharmaceutical market, the vaccine market is relatively small and concentrated on both supply and demand sides. It is highly regulated and largely dependent on public purchasers and donor policies. The vaccine market has very distinct features, which increase the complexity of assessing and understanding pricing and procurement. It is made up of individual markets for individual vaccines or vaccine types, each with their own specificities, particularly on the supply side.69
Individual vaccine markets are constantly changing as countries introduce new vaccines and change preferences for more traditional vaccines. Until recently, only high-income countries (HICs) were purchasing more complex and higher priced products whereas low- and most of the middle-income countries were purchasing more mature vaccines and presentations. Now the dynamic is different: there is more convergence in demand for newer vaccine types and more divergence in demand for mature and combination vaccine types.70
About 80% of global vaccine sales come from five large multi-national corporations (MNC) that were the product of various mergers and acquisitions of pharmaceutical companies over the past decades. While maintaining a strong focus on vaccines for industrialized country markets, MNCs also sell their products in developing countries and emerging markets and participate in Global Health Initiatives. To compete in these markets, MNCs will often outsource and participate in joint-development activities and technological transfers. Research based manufacturers are represented by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA).71
In the 1980s emerging market manufacturers started entering the vaccine market and have assumed a significant role since. Emerging manufacturers play a critical role in the supply of vaccines of developing countries, particularly basic and some combination vaccines. They now supply about half of UNICEF’s vaccine procurement in volume of doses, representing about 30% of the value of UNICEF’s total vaccine procurement.72 The entry of emerging market manufacturers, particularly in the underused vaccines market, has resulted in lower vaccine prices due to increased competition and higher production capacities for individual vaccines. A few emerging market manufacturers are also trying to expand their production to newer vaccines. Emerging manufacturers are represented by the Developing Countries Vaccine Manufacturers Network (DCVMN).73
There are relatively few vaccine manufacturers that meet international standards of quality established by WHO. Many of the individual vaccine markets are monopolies or oligopolies, either by product or presentation.74 The limited number of vaccine suppliers and production capacities leads to a tenuous balance between demand and supply in many individual vaccine markets. Constant management and communication between market actors is absolutely required to guarantee sufficient supply of vaccines for each purchaser.75
The main actors on the demand side of the vaccine market are governments of industrialized and developing countries, pooled procurement agencies, the private sector, and the various regulatory and advisory bodies overseeing vaccine quality and safety.76 High Income Countries (HICs) constitute 82% of global vaccine sales in terms of value, corresponding to about 20% of the annual volume of vaccines sold. Not only do HICs pay higher prices, they are more likely to implement newer vaccines.77
Pooled procurement mechanisms including those operated by UNICEF and PAHO on behalf of LICs and MICs constitute the second major group of vaccine purchasers after high-income countries. Together, low- and middle-income countries account for about 18% of the value of global vaccine sales, constituting approximately 80% of the annual volume sold. In 2011, WHO estimated the combined purchases of UNICEF and PAHO at USD 1.43 billion, equaling of around 7% the value of total vaccine sales.78 UNICEF Supply Division and PAHO Revolving Fund have considerable influence on the market, which allows them to procure vaccines for significantly lower prices than many countries could achieve on their own. Annually UNICEF procures some or all vaccines for up to 100 countries whereas PAHO procures for around 40 member states.79
The number of countries procuring vaccines individually is rather limited. In some cases, individual self-procuring countries are competing for limited supply with large procuring entities.80
With a WHO estimate of 5 to 10% of total vaccine sales in developing countries, the private sector plays in general a relatively small role on the demand side, except in some populated countries with rapid economic growth where demand from middle classes for new and non-EPI vaccines can be of significant value. Private sector demand mainly consists of more affluent population segments in developing countries that decide to take on responsibility for their own immunization, using preferred presentations not offered by the public sector.81
On the demand side, WHO and the national immunization technical advisory and regulatory entities have significant influence in setting the agenda, calendars and immunization policies and programmes which ultimately determine the level of global demand.82
The “Vaccines 2020: World Market Analysis, Players, Trends” examines the market for these vaccines used to prevent various types of disease, and provides market modeling by disease.83 Seven billion dollars in new sales have occurred in the past five years alone, and vaccines have been developed and deployed for new diseases. New vaccines are under development.84
It focuses on commercialized vaccines and developmental vaccines for diseases that are already vaccine-preventable, with a discussion of selected emerging vaccines for diseases that are not currently vaccine-preventable, such as addiction and malaria. The report also discusses trends and looks at what vaccine companies have done and are planning. It also covers the COVID-19 vaccine market landscape, development progress and estimated market opportunity as of October 2020.85
Because of the large number of deadly diseases that have been virtually eliminated through the proliferation of effective vaccines, vaccination is generally viewed as one of the greatest public health achievements during the 20th century. As a result of widespread public vaccination, vaccine-preventable diseases and their resulting deaths are now rare in the developed nations and declining worldwide.86 Immunizations have eradicated smallpox; eliminated poliomyelitis in the Americas; and controlled measles, rubella, tetanus, diphtheria.87
Development of new vaccines is ongoing. Generally speaking, vaccine clinical development in the world’s major markets follows a similar pathway as drugs and other biologics. A sponsor who wishes to begin clinical trials with a vaccine must first obtain permission to conduct clinical studies. In the U.S., this requires the submission of an Investigational New Drug (IND) application to the FDA. The IND describes the vaccine, its method of manufacture, and quality control tests for release. Also included is information about the vaccine’s safety and ability to elicit a protective immune response (immunogenicity) in animal testing as well as the proposed clinical protocol for studies in humans. The report tracks development of vaccines, including those for COVID-19.88
The fight against COVID-19 has seen vaccine development move at record speed, with more than 170 different vaccines in trials. But how are they different from each other and how will they protect us against the disease?89 There are more vaccine candidates simultaneously in the pipeline for COVID-19 than ever before for an infectious disease. All of them are trying to achieve the same thing – immunity to the virus, and some might also be able to stop transmission. They do so by stimulating an immune response to an antigen, a molecule found on the virus. In the case of COVID-19, the antigen is typically the characteristic spike protein found on the surface of the virus, which it normally uses to help it invade human cells.90
There are four categories of vaccines in clinical trials: Whole Virus, Protein Subunit, Viral Vector and Nucleic Acid (RNA and DNA. Some of them try to smuggle the antigen into the body, others use the body’s own cells to make the viral antigen.91
Vaccines stimulate a person’s immune system to protect them from a specific disease, exemplifying the expression: “An ounce of prevention is worth a pound of cure.” Indeed, according to the World Health Organization (WHO), vaccines prevent two million to three million global deaths annually, and could prevent an additional 1.5 million deaths each year with improved access.92
S&P Global Ratings believes the worldwide vaccine market currently provides industry participants steady and healthy revenue growth of about 5%-7% annually, and good profitability supported by significant barriers to entry.93
The vaccine market has attracted a lot of public attention recently, largely due to the coronavirus pandemic, resulting in an investment surge as part of the race to develop a COVID-19 vaccine. Although this could add tens of billions of dollars to industry revenues in 2020-2022, we think it may also accelerate technological innovations and support the ambitions of potential market entrants, leading to more intense competition and margin pressure. Alternatively, it may lead to leverage-harming mergers and acquisitions (M&A) as leading market participants seek to protect their leadership positions.94
Although the vaccine market shares many characteristics with the broader pharmaceutical market, including substantial barriers to entry in the form of high fixed costs; often, long production timelines; substantial intellectual property; high regulatory requirements; and low sensitivity to the business cycle, they differ in notable ways.95
More specifically, market participants can benefit from greater government funding for research and development (R&D), government promotion and purchasing of vaccines, higher revenue growth, a high degree of consolidation and less intense competition, and products with long lifecycles. On the other hand, vaccine manufacturers also have a more consolidated group of buyers, weaker pricing power, and may face elevated risks of disruption from technological advances.96
The differences result in a distinct set of opportunities and key credit risks, and provides the four large pharmaceutical companies that participate in, and dominate, the vaccine industry, an extra element of business diversity. For example, we view the vaccine market as relatively immune to generic competition in developed countries and to potential drug-price reform in the U.S.97
The global vaccine market generated approximately $33 billion of revenue in 2019, which represents less than 3% of the global pharmaceutical market. In contrast, the global market for oncology drugs in 2019 was about $142 billion. The population base of healthy individuals for preventative vaccines is much larger, and represents a younger demographic than for conventional restorative-type medicines. Indeed children receive the majority of vaccines, often focusing on childhood diseases.98
The vaccine industry has experienced robust growth over the past two decades with a compound annual growth rate (CAGR) of more than 6% over the past five years. And, we expect annual growth of about 5%-7% over the next five years, excluding the growth from a COVID-19 vaccine. Market growth is supported by the development of more vaccines addressing new indications; higher prices on new innovative vaccines; increased demand in emerging markets; and the growth of combination vaccines, such as the measles, mumps, rubella, and varicella (MMRV) vaccine. These combined vaccines earn premium pricing by enhancing the efficiency of doctors by addressing multiple indications with the administration of a single injection, facilitating widespread adoption through easier dosing.99
We expect COVID-19 vaccines could potentially generate tens of billions of revenues in 2020-2022, pending regulatory approval, but then potentially taper off, following the initial surge. However, due to uncertainties around pricing (including commitments to limit profits) competition, rate of adoption, and the potentially temporary nature of that initial demand, our discussion of industry growth here, largely excludes the potential impact of COVID-19 products.100
That said, the vaccine market is getting renewed attention as part of the race to develop a COVID-19 vaccine, and a surge of investment may accelerate technological advancements and support the ambitions of potential market entrants including small biotech companies, leading to more intense competition and margin pressure. It may also lead to leverage-harming M&A as leading market participants seek to protect their leadership positions with debt-financed acquisitions.101
Contract development and manufacturers of drug products (CDMOs) are benefitting from the sudden surge in demand for available manufacturing capacity, to support COVID-19 vaccines. Although we expect a portion of that demand to subside once the initial wave of vaccines is available, we see CDMOs having a more sustained benefit as a necessary partner to small biotech companies entering the market that don’t have their own manufacturing capacity, at least as long as those companies remain independent. Moreover pressures on Big Pharma to onshore more production and diversify supply chains resulting from the pandemic, may lead them to increase utilization of CDMOs, over the next several years.102
According to Claire Felter (2021) [a] year into the pandemic of the COVID-19 coronavirus disease, the global effort to develop and distribute an effective vaccine has already produced several promising options. The accelerated development of multiple vaccines is unprecedented; the process typically takes eight to fifteen years.103 Now, the immunization of a critical mass of the world’s population—which is crucial for getting the pandemic under control—is up against a new set of challenges, including dangerous new strains of the virus, global competition over a limited supply of doses, and public hesitation about the vaccines.104
Several vaccines have been approved for general or emergency use in countries including China, Russia, the United Kingdom, and the United States. As of February 2021, over two hundred million doses had been administered worldwide. Several countries—such as Israel and the United Arab Emirates—are making swift progress immunizing their citizens, while the vast majority have either vaccinated only small fractions of their populations or are yet to start.105
Traditionally, vaccines are dead or weakened virus molecules—known as antigens—that trigger defensive white blood cells in the immune system to create antibodies that bind to the virus and neutralize it. There are four main types of conventional vaccines:
- live vaccines use a weakened form of the virus to prompt the creation of antibodies;
- inactivated vaccines use a dead version of the virus;
- toxoid vaccines use toxins made by the virus to produce immunity to the part of the virus that causes disease; and
- subunit, recombinant, polysaccharide, and conjugate vaccines use proteins or other pieces of the virus.
There are also several new types of vaccines that use the virus’s genetic material—DNA or RNA—to prompt the body to create antibodies. More than a dozen of the COVID-19 vaccine candidates that have gone to clinical trials are genetic-based, including those by U.S. pharmaceutical giant Pfizer and partnering German firm BioNTech and by U.S.-based Moderna. No vaccine of this kind had ever been approved for commercial use in humans before the COVID-19 pandemic.106
When most of a population has been vaccinated and is immune to a particular disease, even those who are not immune are considered protected because the likelihood of an outbreak is small. This is known as herd immunity . Chicken pox, measles, mumps, and polio are all examples of diseases for which the United States has achieved herd immunity due to vaccines. Scientists are divided about how much of a population must have COVID-19 antibodies to prevent new outbreaks, with estimates ranging from less than half to over 80 percent.107
Vaccines are frequently collaborative efforts across sectors of society, with private pharmaceutical firms teaming up with public health agencies or university labs. Here are snapshots of some of the major players in the COVID-19 vaccine field.108
- Governments. Public health agencies have played critical roles in supplying funds to develop COVID-19 vaccines. In the United States, President Donald J. Trump’s administration launched Operation Warp Speed, a project aimed at developing an effective vaccine and manufacturing enough doses for all three hundred million Americans. The effort, which pledged billions of dollars to companies with promising candidates, brought together several agencies within the Department of Health and Human Services—including the Centers for Disease Control and Prevention, the National Institutes of Health (NIH), and the Food and Drug Administration (FDA)—and the Department of Defense. The European Commission has also funded several candidates; at a virtual summit in May 2020 hosted by the European Union, world leaders, organizations, and banks pledged $8 billion for vaccine research. In China, the government has closely overseen efforts on its territory, with state-owned firms such as Sinopharm making up about two-fifths of the country’s vaccine industry.109 (Ibid)
- International institutions. The World Health Organization (WHO) and other multilateral institutions such as the World Bank are focused on financing and manufacturing COVID-19 vaccines for global use, in particular to ensure fair allocation among all countries. Also at the forefront of multilateral efforts is the Coalition for Epidemic Preparedness Innovations (CEPI), a global alliance that was founded by Norway, India, the Bill & Melinda Gates Foundation, the UK-based Wellcome Trust, and the World Economic Forum. Gavi, the Vaccine Alliance—also founded by the Gates Foundation—is a public-private partnership focused on improving vaccine access for lower-income countries. In June 2020, the WHO, CEPI, and Gavi launched COVAX, a global initiative aiming to distribute two billion vaccine doses by the end of the following year. By February 2021, COVAX had begun its deliveries, sending doses first to West Africa.110
- Private sector. The pharmaceutical industry has been driving much of the push. Companies ranging from biotech start-ups to giants such as U.S.-based Johnson&Johnson shifted their research and development efforts to focus on COVID-19. While early research into a vaccine candidate typically receives government funding, such as NIH grants in the case of the United States, the bulk of financing for clinical development generally comes from private sources.111
- Research institutions and nonprofits. Many of the COVID-19 vaccine candidates have involved a university or college assisting in preclinical research or clinical trials. In the case of the University of Oxford’s candidate, the research team was already working on vaccines for an unknown disease that could cause a pandemic; then, in January 2020, the group zeroed in on COVID-19. The Gates Foundation has been the leading nonprofit funding COVID-19 vaccine efforts.112
Most of the vaccines approved for at least limited use have been developed by firms and research groups in China, Russia, and the United States. The first human trial in the United States began in Seattle in March 2020 with a vaccine by Moderna Inc. That vaccine was approved for emergency use in the United States, members of the European Union, and several other countries after it appeared highly effective in large-scale trials. A vaccine by Pfizer and BioNTech was also authorized by regulatory agencies in dozens of countries after similarly promising results. Johnson&Johnson’s vaccine was next to enter the U.S. market, receiving emergency authorization in late February 2021. Meanwhile, Beijing has approved four of its candidates, all of which are being used by other nations. Russia approved two vaccines before testing them in large trials; one of these is being distributed in tens of other countries.113
Additionally, countries around the globe have endorsed a vaccine by the UK’s University of Oxford and British-Swedish company AstraZeneca that is cheaper and easier to store and transport than some others.114 Dozens of other COVID-19 vaccine candidates are undergoing large-scale clinical trials and around 180 potential vaccines are in preclinical development by pharmaceutical companies, academic institutions, and government agencies.115
Under normal circumstances, during which the stages of vaccine development occur sequentially, a vaccine takes eight to fifteen years on average to get from the lab into the hands of health-care providers. The fastest a vaccine had ever been developed before this pandemic is four years. Following the emergence of COVID-19, however, researchers around the globe have accelerated the process by carrying out stages of development simultaneously and by looking to new vaccine technologies. “I think what we’re seeing is remarkable,” says Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia. “It is a scientific tour de force.”116
The U.S. Operation Warp Speed timeline hinged on overlapping stages of development; mass production started for strong candidates even while clinical trials were ongoing. Before their vaccines were approved, Moderna received $2.5 billion in a deal under Warp Speed that included the purchase of one hundred million doses, while Pfizer and BioNTech signed a $1.95 billion contract to manufacture and distribute one hundred million doses of their vaccine. (Pfizer executives said they have not accepted any U.S. federal funding for the development of their vaccine.) Shortly after President Joe Biden took office, his administration bought another hundred million doses each from these companies.117
Another way researchers have quickened the process is by focusing on new vaccine approaches. RNA- and DNA-based vaccines can be developed far faster than conventional vaccines, which require months at a time of growing antigens in animal or insect cells.118
Dozens of treatments—which would not prevent someone from being infected with COVID-19 but could help reduce the severity and duration of illness—have been developed or repurposed. Among them is the antiviral drug remdesivir, which was developed by U.S.-based Gilead Sciences and approved by the FDA. An NIH-sponsored trial of remdesivir that involved dozens of sites in the United States, Europe, and Asia showed faster rates of recovery from the virus. Some health experts are also optimistic about the use of dexamethasone, a common steroid, which was found to reduce the risk of death in severely ill COVID-19 patients in the UK. The FDA has authorized emergency use of convalescent plasma, or blood plasma of previously infected people who have created COVID-19 antibodies. Though plasma donations have already been used in tens of thousands of patients, there have not been large, robust studies to determine the treatment’s effectiveness.119
Even with several vaccines approved for emergency use, there remains the tremendous challenge of making enough of them for the world’s population. Though multilateral initiatives such as COVAX and individual governments are investing billions of dollars to expand production plants, current global manufacturing capabilities are far below what’s needed—only about a dozen countries have the capacity to produce COVID-19 vaccines.120
This task has not only motivated countries to scale up production, but also pitted them against one another amid a limited vaccine supply. Wealthy countries including Australia, Canada, and the United States have struck deals with manufacturers to provide their countries with more than enough doses for their populations, leaving lower-income countries unable to immunize but a small proportion of their citizens in the coming months. Brazil, China, and India all have large vaccine industries, which allows them to reserve some of their vaccine supplies for their own residents.121
Experts including CFR’s Thomas J. Bollyky have warned that bidding wars over vaccines lead to inequitable distribution and, ultimately, fail to eliminate the risk of new outbreaks. In a February 2021 op-ed, WHO Director General Tedros Adhanom Ghebreyesus echoed this, writing that leaving large swaths of the global population unprotected is “epidemiologically self-defeating.” However, there have been signs of increasing global cooperation: Group of Seven (G7) nations have committed billions of dollars toward equitable vaccine access, including $4 billion from the United States toward COVAX over the next two years; the UN Security Council also adopted a resolution on unhindered vaccine access in areas of armed conflict.122
Meanwhile, several new strains of the coronavirus are raising concerns among scientists and health officials about increased transmission, waning immunity in people already infected with COVID-19, and reduced effectiveness of vaccines that have already been developed. Pfizer, BioNTech, and Moderna are already developing booster shots to increase protection against the new strains. And in a promising sign ahead of Johnson & Johnson’s U.S. approval, the company reported its vaccine provides higher protection against these strains than initially thought.123
On top of these challenges are the public’s concerns about sped-up vaccines. In a November 2020 poll by the Pew Research Center, roughly 40 percent of Americans surveyed said they would not get a coronavirus vaccine if it were available to them now. “We’ve not done a really good job of saying, ‘Here’s what happens if you get this vaccination and here’s what happens if you don’t’,” says Georges C. Benjamin, executive director of the American Public Health Association. “We’ve not married those two stories in a compelling way for a lot of people who are fundamentally hesitant.”124
Despite the enclave or exclusive nature of, and the internal limitations of the global vaccine market, vaccines are now being collectively seen as the Messiah to deliver the world from the skuldudgeries of the pandemic.
But it has become obvious that attention seems to have fully shifted to vaccination as countries scramble to have their shares of the available vaccines produced by the Big Pharma vaccine manufacturers. No country wants to be seen being left out. The situation presents a scenario of mad rush. There are now several vaccines on the global market shelf from which countries can chose depending on several factors such as medical advisories by health statutory authorities or even whims and caprices of the political leaders. Definitely not all countries are using the same vaccine. Each vaccine manufacturer and country are pushing their vaccines as the best leaving the freedom of choice to each government.
US Goes for the Jugular of Covid-19
Interestingly, it was not the United States that would first apply vaccine against the coronavirus. This was done by China where the virus actually came from. But the degree of success of this vaccination exercise is not known within the overall suite of safety measures taken by Chinese authorities to mitigate the deadly effects of the pandemic. Much has indeed been spoken of Chinese success in largely halting the spread of the pandemic in China in comparison with other countries. But little is known about the contribution of vaccination to this overall success.
According to Jon Cohen, the first people in the world to receive a COVID-19 vaccine were not part of a clinical trial. No TV stations or newspapers covered the historic event. No company issued a statement.125 On 29 February, less than 2 months after the world awakened to the threat of the new disease, virologist Chen Wei, a major general in China’s army, and six military scientists on her team stood in front of a Chinese Communist Party flag and received injections of an experimental COVID-19 vaccine. Chen, a national hero for her work on Ebola vaccines, had come to the initial center of the pandemic, Wuhan, with her group from the Academy of Medical Military Sciences, in part to help make the candidate vaccine with a commercial company, CanSino Biologics. Commentators inside and outside of China later questioned whether the event, which received wide play on social media, was real. No less than People’s Daily, the Communist Party’s main newspaper, labeled a photo of Chen receiving the vaccine as “#FAKENEWS.” But Hou Li-Hua, a researcher at the academy who works on the vaccine project, says it was “true news”—an attempt to protect the scientists in the hard-hit city.126
In the United States, the Trump administration’s $10.8 billion Operation Warp Speed accelerated vaccine R&D faster than many researchers thought possible, specifically for the U.S. population. But an equally massive effort unfolded in China. CanSino and two other Chinese companies—one owned by the government, the other working closely with its regulatory agency—are investing substantial resources, testing four candidates in tens of thousands of volunteers around the world, and are likely only days or weeks away from announcing the outcomes of efficacy trials, just behind the encouraging early results announced over the past month by Pfizer and BioNTech, Moderna, AstraZeneca and the University of Oxford, and Russia’s Gamaleya Research Institute of Epidemiology and Microbiology.127
But the low profile of those historic first injections, the military collaboration with a “private” company, and the ethically fraught decision to start with vaccinations outside of a clinical trial telegraphed that aside from the similar scale and speed, China’s vaccine effort is following a very different course from those in the United States and Europe. Most leading Western vaccines rely on sexy technologies such as genetically engineered viral vectors, designer proteins, and snippets of RNA. Three of China’s four leading vaccine candidates use an unfashionable stalwart: the whole inactivated virus, an approach that dates back to the first successful flu vaccine in the 1930s. And China’s vaccine effort is cursed by its dramatic success with aggressive public health measures to stop the spread of the coronavirus SARS-CoV-2, including forced isolation of cases and testing of entire cities. Whereas the raging pandemic in the United States has enabled trials there to quickly deliver signals of efficacy, “China crushed the coronavirus epidemic early, so they lost the opportunity to test the efficacy of their vaccines there,” says epidemiologist Ray Yip, who closely follows COVID-19 vaccine development as an adviser to Bill Gates. “If they had plenty of cases in China, they could have finished an efficacy trial ahead of other people.”128
So China’s vaccine developers have gone abroad. Although the United States has shut them out of Operation Warp Speed, they have brokered deals with 15 other countries on five continents. They have mounted massive trials in the Arab world—and given candidate vaccines to top government officials there—and navigated toxic politics in Brazil, where the pandemic is raging fiercely, to test a vaccine and explore producing it there.129
But China isn’t just seeking promising venues for clinical trials. Not urgently needing the vaccines at home to fight a virus it has largely quashed, it is playing a global game by pledging to send any proven vaccine to countries that are conducting trials for its candidates, or to share the technologies behind them. “They know they don’t need a vaccine to contain the epidemic in China,” Yip says. “They can take their sweet time.”130
Yanzhong Huang, a global health specialist at both Seton Hall University and the Council on Foreign Relations, says the country is “actually using the vaccine to promote the diplomacy of foreign policy objectives.” This “vaccine diplomacy” he says, contrasts starkly with Warp Speed’s “vaccine nationalism” and aims to “fill in the void left by the United States.”131
“It is a very carefully executed and carefully thought out strategy,” says Stephen Morrison, who directs the Global Health Policy Center at the Center for Strategic & International Studies. “A strategic goal of the Chinese government is to achieve hegemonic influence in the bioeconomy within the next decade.”132
At home, too, attitudes toward vaccines contrast with those in the United States and Europe, where mistrust is high, Morrison says. To the consternation of vaccine experts overseas, hundreds of thousands of people in China have already lined up to receive the experimental vaccines—even before their value and safety have been proved. “There has not been a collapse of faith and trust in science and in the state,” Morrison says. “There’s less fear about where this is all going.”133
But it was none other than the United States that would first go for the jugular of Covid-19 with the help of vaccine on a mass scale at least as widely reported by the media worldwide although in an atmosphere of political acrimony and/or uncertainty. The process of vaccination in the US actually started under Trump Administration in December 2020 when the political atmosphere was still highly charged with claims and counterclaims about the November 3, 2020 Presidential Election.
The first Covid-19 vaccination in the United States took place in New York City on December 14, 2020, as the country gears up for its largest ever immunisation campaign.134 “I feel like healing is coming,” said New York nurse Sandra Lindsay – among the first health workers given the jab.135
While the death toll hit 300,000 around this time, 150 hospitals across the country were been expected to receive millions of vials of the Pfizer/BioNTech vaccine. The US vaccination programme aims to reach 100 million people by April.136 The Pfizer/BioNTech vaccine received emergency-use authorisation from the US Food and Drug Administration (FDA) the previous week Friday. The roll-out comes as the epidemic continues to ravage the country. Deaths have been rising sharply since November and the number of people in hospital with the disease has also continued to grow steadily, with more than 109,000 people currently admitted, according to the Covid Tracking Project.137
The Pfizer/BioNTech vaccine – a collaboration between a US pharmaceutical giant and a German biotechnology company – offers up to 95% protection and is the first Covid-19 vaccine to be approved by US regulators. It is already being rolled out in the UK, while Canada also began its innoculation programme on Monday, with an initial 30,000 doses going to 14 sites across the country.138
The first three million doses in the US are being distributed to dozens of locations across all 50 states by cargo plane and truck. Because the vaccine has to be kept at extremely low temperatures, the vials are stored in dry ice-cooled packages as they are whisked around the country. GPS-enabled thermal sensors are also being used to track the temperature of shipments as they are delivered.139
Most Americans will not be able to receive the vaccine until well into 2021, but the roll-out beginning this week is seen as a key symbolic turning point in the nation’s battle against the pandemic, with hopes that take-up will be high. “I think it’s been probably the darkest December on record here. As of last week, Covid-19 is the leading cause of death in the US, even more than cancer and heart disease,” Dr Dora Mills of MaineHealth, a network of 12 hospitals in Portland, Maine, told the BBC. “It’s a very dark season for us, but it’s also extraordinary that we have a vaccine less than a year after this virus has emerged. If the efficacy and safety data hold up, this is likely [to be] the greatest public health and scientific achievement of our lifetime.”140
[Former] US Health Secretary Alex Azar told NBC’s Today programme there was now a “light at the end of the tunnel of this horrible pandemic”. “If you are recommended to get it, and it’s available to you – oh, please do get it,” he urged. “Protect yourself and protect those around you. But please, get the vaccine.”141
The first doses are expected to be given to selected healthcare workers and elderly people living in residential care. Health centres are planning to stagger vaccinating workers to account for any side effects, according to US media. The reported side effects, including fatigue, fever and headaches, are typically mild to moderate in severity. Researchers say any serious side effects are rare. Senior members of the Trump administration had been due to be some of the first in line, but President Trump said he had reversed that plan. He tweeted on Sunday that people working at the White House “should receive the vaccine somewhat later… unless specifically necessary”.142
[Meanwhile] US President-elect Joe Biden, who will be inaugurated as president on 20 January, has set a goal of 100 million Covid vaccinations in his first 100 days in office. That would represent roughly a third of the country’s total population. Pfizer has agreed a deal to supply the US with 100 million doses of the vaccine by March. An additional 200 million doses of a second vaccine, developed by Moderna and the National Institutes of Health, will be provided by June.143
Moderna was the second Covid-19 vaccine to be approved by the US government, clearing the way for millions of doses to be released.144 The Food and Drug Administration (FDA) authorised the US-made jab about a week after approving a Pfizer/BioNTech vaccine which is now being distributed. The US has agreed to purchase 200 million doses of Moderna, and six million may be ready to ship now.144
FDA commissioner Stephen Hahn said the emergency approval of the vaccine on Friday marked “another crucial step in the fight against this global pandemic that is causing vast numbers of hospitalizations and deaths in the United States each day”.145 The authorisation came after an advisory panel on Thursday voted 20-0 with one abstention that the benefits of the Moderna vaccine outweighed the risks for those aged 18 and over. Regulators reported earlier this week that the Moderna vaccine was safe and 94% effective.146
US President Donald Trump, who hours before the official announcement tweeted that the vaccine had been “overwhelmingly approved” and distribution would “start immediately”, said on Twitter: “Congratulations, the vaccine is now available” [But] President-elect Joe Biden, who is set to be vaccinated on Monday, said the authorisation of the Pfizer and the Moderna jabs “assures us that brighter days lie ahead”. But, he added, “the fight against Covid-19 is not yet over.” “We know the immense challenges ahead, including scaling up manufacturing, distribution, and the monumental task of vaccinating hundreds of millions of Americans. We need to make sure we have the resources to do all of this and to do it quickly.”147
How does Moderna differ from the Pfizer vaccine? It requires temperatures of around -20C for shipping – similar to a normal freezer. The jab manufactured by US corporation Pfizer and Germany’s BioNTech SE requires temperatures closer to -75C, making transport logistics much more difficult. Like the Pfizer jab, the Moderna vaccine also requires a second booster shot. Moderna’s second injection comes 28 days after the first, compared with 21 for Pfizer.148
Moderna is based in Cambridge, Massachusetts, and had previously said that the “vast majority” of its doses would be manufactured there. Pfizer/BioNTech is being manufactured in several countries, including Germany and Belgium.149
Other countries have also ordered the Moderna vaccine:
- Canadaplans to get two million doses by March – part of a total 56 million doses
- The UK has already pre-ordered seven million doses
- The European Union last month announced a contract to purchase of 80 million doses – with an option to purchase up to 80 million more – once the vaccine is deemed safe and effective
- Japan has signed up for 50 million doses, South Korea for 20 million, and Switzerland has ordered 7.5 million, according to data compiled by the Duke University Global Health Innovation Center.150
Centers for Disease Control and Prevention guidelines submitted to US states say healthcare workers should be prioritised first, as well Americans living in long-term care homes. Essential workers are expected to be next in line for the jab, but it will be up to states to decide which industries to prioritise. Moncef Slaoui, the chief scientist of federal vaccine distribution programme Operation Warp Speed, says the young and healthy should be at the back of the queue. At least 70% or 80% of the US population need to be vaccinated to achieve herd immunity, he said.151
In a statement, top US infectious disease expert Anthony Fauci said he hoped that “all Americans will protect themselves by getting vaccinated when the vaccine becomes available to them”. “That is how our country will begin to heal and move forward,” he said.152
Forward Match with Vaccination
Despite the slow roll-out of COVID-19 vaccination in the U.S., the pace of vaccination has begun to pick up across the country. U.S. vaccine supply is running at about 11 million per week, with an average of 1.7 million doses being administered per day; shots are now getting into people’s arms as fast as they’re becoming available. This is in part due to the easing of vaccine supply constraints, as the Biden administration has released more doses and provided more predictability to states, and production schedules have sped up. Still, the number of people eligible in states is increasing, and these next few weeks will be critical ones in the race to vaccinate as many people as possible, due to continued spread of COVID-19 and the emergence of viral variants. Given what we know about projected supply, where could we be with vaccination by the end of the first quarter of this year (March 31)?153
To answer this question, we looked at the number of doses estimated to be delivered by Pfizer and Moderna, the two manufacturers with authorized vaccines in the U.S., between now and the end of March. The U.S. has purchased 600 million doses (300 million from each company) which, given the two-dose regimen required by these vaccines, is enough to vaccinate 300 million people. Even without the addition of any new vaccine, this would go far in achieving U.S. population immunity (though it is widely expected that the FDA will authorize Johnson&Johnson’s one shot vaccine soon, increasing supply to more than enough to reach the entire U.S. population).154
Pfizer has said that it expects to deliver 120 million doses to the U.S. by the end of March, 20 million ahead of its original schedule. Moderna has said it will deliver 100 million doses. Assuming these production schedules hold, that means that 220 million doses, enough to fully vaccinate 110 million people, will be delivered within a month and a half from today.155
Figure 1: Estimated Average Daily Doses Administered in the United States: Current and Projected
As at February 17, 2021, [t]he seven-day average of coronavirus vaccines administered in the U.S. has reached 1.7 million per day, White House coronavirus coordinator Jeff Zients said at a Wednesday briefing.156 That pace puts President Biden on course for meeting his goal of 100 million doses administered in his first 100 days in office, which would land on April 29. 54 million vaccine shots have been administered thus far, and 5% of Americans have received both doses.
- If the federal government — and states handling daily vaccine operations — can maintain this course of 1.7 million shots per day, 80% herd immunity could be reached by Nov. 17, according to a Washington Post analysis
- Speeding up the rate of vaccinations is key in order to get life back to some semblance of normal and prevent potentially vaccine-resistant variants from becoming dominant in the U.S., per the Post.
The White House weekly vaccination progress report displayed at Wendesday’s briefing.157
Zients told governors Tuesday that beginning this week, the federal government’s weekly allocation of vaccines to states will increase from 11 million doses to 13.5 million doses — a total increase of 57% since the start of the Biden administration.
- The administration is also doubling the weekly supply of vaccine doses to local pharmacies from 1 million to 2 million, and has activated 1,200 National Guard troops to serve as community vaccinators .
- Zients said that community centers, high school gyms, churches, and stadiums are serving as vaccination sites, with federally-run sites “that can give over 30,000 shots a week.”
The U.S. is on track to have enough vaccine supply for 300 million Americans by the end of July, according to Zients.158
According to Bloomberg, [m]ore than 334 million doses have been administered across 121 countries, according to data collected by Bloomberg. The latest rate was roughly 8.41 million doses a day. In the US, 98.2 million doses have been administrated as at March 11, 2021.159
The average number of vaccine doses being administered across the United States per day topped two million for the first time on Wednesday, according to data from the Centers for Disease Control and Prevention. A month ago, the average was about 1.3 million.160
Mr. Biden has also promised to administer 100 million vaccines by his 100th day in office, which is April 30. As of Thursday, 54 million people have received at least one dose of a Covid-19 vaccine. Johnson & Johnson’s one-shot vaccine was authorized for emergency use on Saturday, but those doses do not appear yet in the C.D.C. data.161
The milestone was yet another sign of momentum in the nation’s effort to vaccinate every willing adult, even as state and city governments face several challenges, from current supply to logistics to hesitancy, of getting all of those doses into people’s arms.162 Mass vaccination sites across the country are opening up or increasing their capacity, in part to respond to the new influx of doses from Johnson & Johnson.163
The Federal Emergency Management Agency has recently helped open seven mega-sites in California, New York and Texas, that are staffed with active-duty troops. In Chicago, a vaccination site at the United Center will open next week, with a capacity of 6,000 shots a day. Many more such sites are planned.164
There have been some hiccups in the massive logistical challenge of distributing millions of doses across the country, with special requirements for storage and handling. In Texas, more than 2,000 doses went to waste over the past two weeks, according to an analysis by The Houston Chronicle. A majority of those losses were blamed on blackouts that swept the state in February, leaving millions of homes and businesses without power, some for multiple days.165
And Mr. Biden has made equity a major focus of his pandemic response, saying he wants pharmacies, mobile vaccination units and community clinics that help underserved communities to help increase the pace of vaccinations. Experts say that Black and Latino Americans are being vaccinated at lower rates because they face obstacles like language barriers and inadequate access to digital technology, medical facilities and transportation. But mistrust in government officials and doctors also plays a role and is fed by misinformation that is spread on social media. In cities across the country, wealthy white residents are lining up to be vaccinated in low-income Latino and Black communities.166
The president said on Tuesday that the country would have enough doses available for every American adult by the end of May, though he said it would take longer to inoculate everyone and he urged people to remain vigilant by wearing masks.167 The administration also announced it had brokered a deal in which the drug giant Merck & Co. will help manufacture the new Johnson&Johnson vaccine. The unusual agreement between two rivals in the pharmaceutical industry was “historic,” Mr. Biden said on Tuesday. “This is a type of collaboration between companies we saw in World War II.”168 Mr. Biden was also going to invoke the Defense Production Act, a Korean War-era law, to give Johnson & Johnson access to supplies for manufacturing and packaging vaccines.169
Meanwhile more than 23 million people in the UK have received at least one dose of a coronavirus vaccine – part of the biggest inoculation programme the country has ever launched.170 In a race against a faster-spreading variant of the virus, ministers have pinned their hopes of easing a third national lockdown on vaccinating as many adults as possible by summer.171 The UK government aims to offer a first vaccine dose to about 32 million people in nine priority groups by 15 April.172 The programme in England is now inviting those aged 56 and above to book appointments after the first four groups – those aged 70 and over, care home residents, healthcare workers and people required to shield – were offered a jab by mid-February. The rest of the over-50s will follow. These groups account for 88% of deaths so far.173
Over 10% of the entire US population has been fully vaccinated for the coronavirus, according to data from the Centers for Disease Control and Prevention.174 More than 33 million people have received either both doses of the Pfizer and BioNTech or Moderna vaccines or a single dose of the Johnson & Johnson vaccine. Roughly 64 million people have received at least one dose of a vaccine.175 With more than 29 million documented cases of the coronavirus in the U.S., more people have now been fully vaccinated than have been reportedly infected with the virus.176
The U.S. is averaging over 2 million shots per day. Last week, the country crossed the threshold of having 10% of the adult population fully vaccinated. The CDC this week recognized the growing number of vaccinated people by issuing new guidance on activities they can resume. “With more and more people getting vaccinated each day, we are starting to turn a corner,” CDC Director Rochelle Walensky said at a Monday press briefing. “And as more Americans are vaccinated, a growing body of evidence now tells us that there are some activities that fully vaccinated people can resume at low risk to themselves.”177
Fully vaccinated individuals who have waited two weeks after their last dose can gather indoors with other fully vaccinated people without masks or physical distancing, according to the guidance. They can also gather withouts masks indoors with unvaccinated individuals from a single household who are not at high risk for severe COVID-19.178 However, they should continue wearing masks in public, Walensky said, because it is not yet known whether they can be mildly or asymptomatically infected and spread the virus.179
On February 26, 2021, the U.S. Food and Drug Administration announced the following actions taken in its ongoing response effort to the COVID-19 pandemic:
- Today, the FDA held a meeting of the Vaccines and Related Biological Products Advisory Committee to discuss the Emergency Use Authorization (EUA) request from Janssen Biotech Inc. for its COVID-19 Vaccine for the prevention of COVID-19 in individuals 18 years of age and older.
- Today, the FDA added new devices to the device discontinuance list, including sterilization products and oxygen conservers. There are no updates to the device shortage list at this time. The FDA will continue to update the device discontinuance and device shortage lists as the COVID-19 public health emergency evolves.
- The FDA’s Office of Medical Policy within the Center for Drug Evaluation and Research presented about the role of the CURE Drug Repurposing Ecternal Link Disclaimer in drug repurposing for neglected tropical diseases as well as COVID-19 at an Internatioanl Society of Neglected Tropical Diseases online Link Disclaimer on February 24. The CRDC is a public-private partnership among C-Path, FDA and the National Center for Advancing Translational Sciences (NCATS), part of the National Institutes of Health. The CDRC is designed to capture real-world clinical outcome data to advance drug repurposing and inform future clinical trials for diseases of high unmet medical need.
- The FDA continues to warn consumers about alcohol-based hand sanitizers that are being packaged in containers that may appear as food or drinks and may put consumers at risk of serious injury or death if ingested. The agency has discovered that some hand sanitizers are being packaged in beer cans, children’s food pouches, water bottles, juice bottles and vodka bottles. Manufacturers should be vigilant about avoiding packaging and marketing their hand sanitizers in containers resembling food or drink packages in an effort to mitigate any potential inadvertent use by consumers. The agency added some of these products to the do not use list of hand sanitizers and urges consumers to be cautious of hand sanitizer packaging. Hand sanitizer can be toxic when ingested. Drinking only a small amount of hand sanitizer is potentially lethal to a young child, who may be attracted by a pleasant smell or brightly colored bottle of hand sanitizer. The FDA continues to monitor these products and will take appropriate actions as needed to protect the health of Americans.
- The FDA published a new web page, COVID-19 Vaccination – the Food and Agriculture Sector , to share information and resources to help employers in the Food and Agriculture sector communicate about COVID-19 vaccination to their workforce. The resources are from the FDA, the Centers for Disease Control and Prevention (CDC), and other trusted partners.
- Testing updates:
- As of today, 332 tests and sample collection devices are authorized by the FDA under emergency use authorizations (EUAs). These include 248 molecular tests and sample collection devices, 70 antibody tests, and 14 antigen tests. There are 37 molecular authorizations that can be used with home-collected samples. There is one molecular prescription at-home test, one antigen prescription at-home test, and one over-the-counter (OTC) at-home antigen test.180
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.181
The same day, (i.e. February 26, 2021), according to a joint statement from the Acting Commissioner of Food and Drugs – Food and Drug Administration, Janet Woodcock M.D. and Director – Center for Biologics Evaluation and Research (CBER), Peter Marks M.D., PhD. “Following today’s positive advisory committee meeting outcome regarding the Janssen Biotech Inc. COVID-19 Vaccine, the U.S. Food and Drug Administration has informed the sponsor that it will rapidly work toward finalization and issuance of an emergency use authorization. The agency has also notified our federal partners involved in vaccine allocation and distribution so they can execute their plans for timely vaccine distribution.182 The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.183
On February 27, 2021, the US Food and Drug Administration authorised the third vaccine to be injected in the US, according to FDA News Release: “Today, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the third vaccine for the prevention of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The EUA allows the Janssen COVID-19 Vaccine to be distributed in the U.S for use in individuals 18 years of age and older.184 “The authorization of this vaccine expands the availability of vaccines, the best medical prevention method for COVID-19, to help us in the fight against this pandemic, which has claimed over half a million lives in the United States,” said Acting FDA Commissioner Janet Woodcock, M.D. “The FDA, through our open and transparent scientific review process, has now authorized three COVID-19 vaccines with the urgency called for during this pandemic, using the agency’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization.”185
The FDA has determined that the Janssen COVID-19 Vaccine has met the statutory criteria for issuance of an EUA. The totality of the available data provides clear evidence that the Janssen COVID-19 Vaccine may be effective in preventing COVID-19. The data also show that the vaccine’s known and potential benefits outweigh its known and potential risks, supporting the company’s request for the vaccine’s use in people 18 years of age and older. In making this determination, the FDA can assure the public and medical community that it has conducted a thorough evaluation of the available safety, effectiveness and manufacturing quality information.186
The Janssen COVID-19 Vaccine is manufactured using a specific type of virus called adenovirus type 26 (Ad26). The vaccine uses Ad26 to deliver a piece of the DNA, or genetic material, that is used to make the distinctive “spike” protein of the SARS-CoV-2 virus. While adenoviruses are a group of viruses that are relatively common, Ad26, which can cause cold symptoms and pink eye, has been modified for the vaccine so that it cannot replicate in the human body to cause illness. After a person receives this vaccine, the body can temporarily make the spike protein, which does not cause disease, but triggers the immune system to learn to react defensively, producing an immune response against SARS-CoV-2.187
“After a thorough analysis of the data, the FDA’s scientists and physicians have determined that the vaccine meets the FDA’s expectations for safety and effectiveness appropriate for the authorization of a vaccine for emergency use,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “With today’s authorization, we are adding another vaccine in our medical toolbox to fight this virus. At the same time, the American people can be assured of the FDA’s unwavering commitment to public health through our comprehensive and rigorous evaluation of the data submitted for vaccines to prevent COVID-19.”188
The Janssen COVID-19 Vaccine is administered as a single dose. The available safety data to support the EUA include an analysis of 43,783 participants enrolled in an ongoing randomized, placebo-controlled study being conducted in South Africa, certain countries in South America, Mexico, and the U.S. The participants, 21,895 of whom received the vaccine and 21,888 of whom received saline placebo, were followed for a median of eight weeks after vaccination. The most commonly reported side effects were pain at the injection site, headache, fatigue, muscle aches and nausea. Most of these side effects were mild to moderate in severity and lasted 1-2 days.189
As part of the authorization, the FDA notes that it is mandatory for Janssen Biotech Inc. and vaccination providers to report the following to the Vaccine Adverse Event Reporting System (VAERS) for Janssen COVID-19 Vaccine: serious adverse events, cases of Multisystem Inflammatory Syndrome and cases of COVID-19 that result in hospitalization or death.190 It is also mandatory for vaccination providers to report all vaccine administration errors to VAERS for which they become aware and for Janssen Biotech Inc. to include a summary and analysis of all identified vaccine administration errors in monthly safety reports submitted to the FDA.191
The effectiveness data to support the EUA include an analysis of 39,321 participants in the ongoing randomized, placebo-controlled study being conducted in South Africa, certain countries in South America, Mexico, and the U.S. who did not have evidence of SARS-CoV-2 infection prior to receiving the vaccine. Among these participants, 19,630 received the vaccine and 19,691 received saline placebo. Overall, the vaccine was approximately 67% effective in preventing moderate to severe/critical COVID-19 occurring at least 14 days after vaccination and 66% effective in preventing moderate to severe/critical COVID-19 occurring at least 28 days after vaccination.192 Additionally, the vaccine was approximately 77% effective in preventing severe/critical COVID-19 occurring at least 14 days after vaccination and 85% effective in preventing severe/critical COVID-19 occurring at least 28 days after vaccination.193
There were 116 cases of COVID-19 in the vaccine group that occurred at least 14 days after vaccination, and 348 cases of COVID-19 in the placebo group during this time period. There were 66 cases of COVID-19 in the vaccine group that occurred at least 28 days after vaccination and 193 cases of COVID-19 in the placebo group during this time period. Starting 14 days after vaccination, there were 14 severe/critical cases in the vaccinated group versus 60 in the placebo group, and starting 28 days after vaccination, there were 5 severe/critical in the vaccine group versus 34 cases in the placebo group.194
At this time, data are not available to determine how long the vaccine will provide protection, nor is there evidence that the vaccine prevents transmission of SARS-CoV-2 from person to person.195
On the basis of the determination by the Secretary of the Department of Health and Human Services on Feb. 4, 2020, that there is a public health emergency that has a significant potential to affect national security or the health and security of United States citizens living abroad, and issued declarations that circumstances exist justifying the authorization of emergency use of unapproved products, the FDA may issue an EUA to allow unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent COVID-19 when there are no adequate, approved, and available alternatives.196
The issuance of an EUA is different than an FDA approval (licensure) of a vaccine, in that a vaccine available under an EUA is not approved. In determining whether to issue an EUA for a product, the FDA evaluates the available evidence to determine whether the product may be effective and also assesses any known or potential risks and any known or potential benefits If the product meets the effectiveness standard and the benefit-risk assessment is favorable, the product is made available during the emergency. Once a manufacturer submits an EUA request for a COVID-19 vaccine to the FDA, the agency then evaluates the request and determines whether the relevant statutory criteria are met, taking into account the totality of the scientific evidence about the vaccine that is available to the FDA.197
The EUA also requires that fact sheets that provide important information, including dosing instructions, and information about the benefits and risks of the Janssen COVID-19 Vaccine, be made available to vaccination providers and vaccine recipients.198
Janssen Biotech Inc. has submitted a pharmacovigilance plan to the FDA describing its commitment to monitor the safety of Janssen COVID-19 Vaccine. The pharmacovigilance plan includes a plan to complete longer-term safety follow-up for participants enrolled in ongoing clinical trials. The pharmacovigilance plan also includes other activities aimed at monitoring the safety profile of the Janssen COVID-19 Vaccine and ensuring that any safety concerns are identified and evaluated in a timely manner.199
The FDA also expects manufacturers whose COVID-19 vaccines are authorized under an EUA to continue their clinical trials to obtain additional safety and effectiveness information and pursue approval (licensure).200
The EUA for the Janssen COVID-19 Vaccine was issued to Janssen Biotech Inc., a Janssen Pharmaceutical Company of Johnson & Johnson. The authorization will be effective until the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biologics for prevention and treatment of COVID-19 is terminated. The EUA for Janssen COVID-19 Vaccine may be revised or revoked if it is determined the EUA no longer meets the statutory criteria for issuance.201
The Centers for Disease Control and Prevention (CDC) said on Thursday [March 11, 2021] about 64.1 million people have received at least one dose of a Covid-19 vaccine, including about 33.9 million people who have been fully vaccinated by Johnson & Johnson’s single-dose vaccine or the two-dose series made by Pfizer-BioNTech and Moderna.202 The work to distribute the vaccine comes as more than 529,000 people in the United States have died after contracting the virus.203 Providers are administering about 2.23 million doses per day on average.204
President Biden has promised to administer 100 million vaccines by his 100th day in office. He set a goal shortly after taking office for the United States to administer more than 1.5 million doses a day, despite criticism that it was not ambitious enough.205 Federal regulators have given emergency approval to vaccines developed by Pfizer-BioNTech and Moderna. Both of those vaccines require patients to receive two doses spaced weeks apart. Regulators authorized Johnson&Johnson’s one-dose vaccine on Feb. 27.206 The federal government has delivered about 131.1 million doses to states, territories and federal agencies.207
Some experts have estimated that 70 to 90 percent of the population needs to acquire resistance to the coronavirus to reach herd immunity, when transmission of the virus substantially slows because enough people have been protected through infection or vaccination.208
A number of factors will determine how quickly this threshold is met, especially the pace at which newly vaccinated people join those who are immune after past infections. But the presence of more transmissible virus variants could complicate that progress.209
If the country maintains its current pace of vaccinating people, about half of the total population would be at least partially vaccinated around late May, and nearly all around late August, assuming supply pledges are met and vaccines are eventually available to children.210
While Covid-19 continue to spread around the world, with more than 114 million confirmed cases and 2.5 million deaths across nearly 200 countries, so also is vaccination across the world progressing uninterrupted except with the emergence of new variants that have surfaced in some countries and spread to others. Vaccination has taken the central stage of activities combating the pandemic in the belief that it is perhaps the key to halting the pandemic and protecting the citizens against further infections. Other factors hitherto held to be responsible for the declining pandemic especially in the US are already been relegated to the background in the thought process about the pandemic.
According to BBC News [t]he US, India and Brazil have seen the highest number of confirmed cases, followed by Russia, the UK and a number of European countries. Very few places have been left untouched. Confirmed cases have been rising steeply since the middle of last year, but the true extent of the first outbreaks in 2020 is unclear because testing was not then widely available. The 100 millionth coronavirus case was recorded at the end of January – about a year after the first officially diagnosed case of the virus.211 Deaths have also been rising, however official figures may not fully reflect the true number in many countries. Data on excess deaths, a measure of how many more people are dying than would be expected based on the previous few years, may give a better indication of the actual numbers in many cases.212
[Meanwhile] several coronavirus vaccines against the virus have now been approved for use, either by individual countries or groups of countries, such as the European Union and the World Health Organization (WHO). Of the 101 countries and territories administering vaccines and publishing rollout data, 60 are high-income nations, 41 are middle-income and none are low-income. The map below, using figures collated by Our World in Data – a collaboration between Oxford University and an educational charity – shows the total number of doses given per 100 people, mostly first doses.213 Overall, the US and China have given the most doses, 68 million and 41 million respectively, while the UK has administered more than 20 million so far. But when breaking the figures down by population, looking at doses administered per 100 people in the 10 countries giving the most vaccinations, Israel, the UAE and the UK top the list – as the chart below shows.214 Most countries are prioritising the over-60s, health workers and people who are clinically vulnerable. Some countries have secured more vaccine doses than their populations need, while other lower-income countries are relying on a global plan known as Covax, which is seeking to ensure everyone in the world has access to a vaccine. Several African countries have now received vaccines through the Covax initiative 215
When the vaccination process started in December 2020, there was no known central coordinating body administering the entire exercise. This is probably because Trump Administration was already visibly in disarray, with serving officials exercising little or no influence on course of events as subordinates threw up their hands helplessly over the increasing chicaneries of President Donald Trump. There was increasing tension in Washington DC as the day of inauguration approaches with barely concealed suspicion that the Inuaguration might be scuttled by President Donald Trump and his core jackals of supporters. The city including several state capitals finally exploded with tension unheard of in the contemporary history of the United States on January 6, 2021 when these jackals trooped out to invade the Capitol to protest against the impeachment of President Donald Trump by the US House of Representatives and prevent the swearing-in of President Joe Biden on January 20, 2021.
The fallouts of this mass protest by the legion of supporters of Trump did not, of course, have any overwhelming influence on their main goal of scuttling the inauguration but rather on the process of vaccination which inexorably slowed down visibly – until President Joe Biden and Vice President Kamala Harris took the mantle of political leadership immediately after the swearing-in on January 20, 2021. Some of the protesters have since been arrested and a put to trial.
The Department of Health and Human Services, even Federal Drugs Administration and other relevant statutory health regulatory bodies all seem to have flunk on their main responsibilities during this period. The former Health and Human Services Secretary, Alex Azer II went to Taiwan leading the highest government delegation to the island State in early August 2020 to learn the secrets of success by Taiwan in defeating the pandemic. But Azer and his entourage came back seemingly empty-handed, having nothing to show for the trip while the pandemic in the US was increasing at alarming rate.
It was the vacuum created by Trump Administration by its abdication of its responsibilities towards the health of the citizens that Joe Biden now took over on January 20, 2021
Revamping the Prostrate Economy with Jumbo Palliatives on Elephant’s Back
Covid-19 came like a thief in the night, murdering members of the household, stealing the peace of the citizens and robbing them of their economic livelihoods. This was more devastating in the United States than any other country in the world. The pandemic cost the United States about 20 million jobs within the space of one year (2020) under the Trump Administration despite the arguments or sophistries to the contrary by former President Donald Trump and his legion of unapologetic supporters.
The Presidential election of November 3, 2020, was therefore not only fought on the basis of the prevalent coronavirus pandemic ravaging the country but also the collateral damages caused by economic crisis as a result of the rampaging pandemic.
President Joe Biden built his main campaign themes around the coronavirus pandemic, revamping the economy depressed by the pandemic and healing a divided nation as a result of racial discrimination exercabated by the pandemic. Of course, issues of foreign policies and national security concerns including other issues of national concern are not left out of the loop. In short, Biden’s outstanding victory was a direct product of the time in which United States and indeed the entire world find itself as a result of the singular issue of the outbreak of coronavirus pandemic. In other words, if coronavirus pandemic has not happened, the election would have taken on a different form or shape and the result would have also been different from what it turned out to be. President Joe Biden is a “child” of circumstances.
Biden Administration approached the pandemic not by sophistries or ideological rhetorics but by concrete policy actions. The first of such proactive actions was the humongous stimulus package of $1.9 trillion. This package can be analogously compared to the Marshal Plan to rebuild Europe after the carnage of the Second World War or the Roseveltian New Deal to save the US economy from total destruction that the modern crisis of capitalism brought it in the 40s.
It can also be compared with the massive bailout for the top-cloistered US banks during the global financial crisis of 2007/2008 by Barack Obama Administration of which Joe Biden was the Vice President then. Obama Administration first came out with $700 billion bailout later augmented by another $831 billion stimulus package of tax cuts and spendings at a time when unemployment peaked at 26-year high of 10 percent then. The recession caused by the global financial crisis ended in June 2009 becoming evident in the summer. GDP growth reached 5 percent in the Fourth Quarter of 2009 which, however, declined to 2.0 percent by the Third Quarter of 2010 with unemployment peaking 9.6 percent in October 2010.
The stimulus package brought to the fore the entire false claims by propaganda by Trump Administration about its economic relief for the high numbers of citizens rendered economically helpless by the pandemic. The so-called economic relief package by Trump Administration did little if at all to mitigate the economic crisis caused by the pandemic. In short, it did not rescue any citizen from the gripping clutches of unemployment escalated to the high heavens by the coronavirus pandemic.
The stimulus package of $1.9 trillion was unveiled on January 14, 2021, even before he was sworn in as the President. “United States President-elect Joe Biden has unveiled a $1.9 trillion stimulus package proposal designed to jump-start the economy and speed up the US response to the coronavirus pandemic.”216 Biden campaigned last year [2020] on a promise to take the pandemic more seriously than President Donald Trump and the package aims to put that pledge into action with an influx of resources for the coronavirus response and economic recovery.
Biden Administration acknowledges that COVID-19 pandemic has forced the United States economy into an economic crisis. Across the country, more than 10 million Americans are unemployed, 14 million renters are behind on payments, and 29 million adults – and at least 8 million children – are struggling with food insecurity. Because of pervasive systemic racism and inequality in our economy, the burdens of this economic crisis are hitting communities of color and other underserved families hardest. One in ten Black workers and one in eleven Latino workers are unemployed. Navigating through the current crisis and emerging stronger requires immediate action to provide equitable economic relief to working families everywhere.217
[Thus] President Biden unveiled a historic legislative package designed to change the course of the pandemic, get students back to school, give families and businesses a bridge to an economic recovery, and invest in advancing racial equity. His plan came on the heels of December’s bipartisan deal to provide a down payment on long-term economic relief for working families. Congress should finish the job by expeditiously passing the American Rescue Plan into law. But the American people cannot afford to wait for Congress to act – they need help and they need it now.218
The actions taken as part of this effort will provide relief to millions of American workers who have lost their jobs and had their hours or wages slashed through no fault of their own. They will help working families feed their children and keep a roof over their head. They will help ensure that unemployed Americans no longer have to choose between paying their bills and keeping themselves and their families safe from COVID-19 by clarifying that workers who refuse unsafe working conditions can still receive unemployment insurance. And, they will help more unemployed workers pay for training and college so they can find better jobs and succeed in an increasingly competitive job market.219
The whole-of-government effort will:
- Address the growing hunger crisis facing 29 million Americans — and as many as 12 million children – by asking the U.S. Department of Agriculture to consider expanding and extending federal nutrition assistance programs.
- Ensure equitable and effective delivery of direct payments — by asking the Treasury Department to consider changing its delivery structure and focus on getting relief to the 8 million Americans who still have not received the financial assistance to which they are entitled.
- Help approximately 2 million veterans maintain their financial footing by asking the U.S. Department of Veterans Affairs to consider pausing federal collections on overpayments and debts.
- Help ensure that unemployed Americans no longer have to choose between paying their bills and keeping themselves and their families safe from COVID-19 by asking the U.S. Department of Labor to consider clarifying that workers who refuse unsafe working conditions can still receive unemployment insurance.
- Enable effective and equitable distribution of government assistance by establishing an interagency benefit coordination structure.220
While additional congressional action is urgently needed to help working families through the remainder of the crisis, these emergency measures are important steps to give millions of Americans real relief during the pandemic. This executive order, combined with the President’s historic relief package and forthcoming jobs package will help Americans persevere through the pandemic and lay the foundation for a strong and equitable recovery. The President is also recommending immediate action to improve the wages, benefits, and bargaining rights of federal workers and contractors.221
As many as 29 million American adults as well as 12 million children are reported to be facing hunger across the country.222 Across the country 1 in 7 household, and more than 1 in 5 Black and Latino households, report that their household is struggling to secure the food they need. In December, Congress bolstered food assistance programs and provided new funding for food banks and school and child care meals. But these measures alone will not solve the growing hunger crisis in America. As part of his American Rescue Plan proposal, President Biden is calling on Congress to provide additional support to ensure that all Americans, regardless of background, have access to healthy, affordable groceries by extending the 15% Supplemental Nutrition Assistance Program (SNAP) benefit increase, investing $3 billion to help women, infants and children get the food they need, and other key steps. The President is also asking the U.S. Department of Agriculture (USDA) to consider taking the following steps to provide nutrition assistance to working families, including to:
- Increase access to nutritious food for millions of children missing meals due to school closures. Established under Families First Coronavirus Response Act, the Pandemic Electronic Benefits Transfer (P-EBT) connects low-income families with kids with food dollars equivalent to the value of the school meals missed due to COVID-related school closures. To date, the program has only allowed P-EBT benefit amounts up to $5.70 per child per school day and many households have had trouble claiming benefits. To address these concerns and expand needed relief, the President is asking USDA to consider issuing new guidance increasing P-EBT benefits by approximately 15% to accurately reflect the costs of missing meals and make it easier for households to claim benefits. For instance, this action could provide a family with three children more than $100 of additional support every two months.
- Allow larger emergency Supplemental Nutrition Assistance Program allotments for the lowest-income households. Congress authorized emergency increases to SNAP benefits to help address food insecurity during the pandemic. So far, those benefit increases have not been made available to all of the lowest income households. USDA will consider issuing new guidance that would allow states to increase SNAP emergency allotments for those who need it most. This would be the first step to ensuring that an additional 12 million people get enhanced SNAP benefits to keep nutritious food on the table.
- Update food assistance benefits to reflect the true cost of a basic healthy diet. More than 40 million Americans count on SNAP to help put food on the table. Currently, however, USDA’s Thrifty Food Plan, the basis for determining SNAP benefits, is out of date with the economic realities most struggling households face when trying to buy and prepare healthy food. As a result, the benefits fall short of what a healthy, adequate diet costs for many households. Therefore, as directed by the 2018 Farm Bill, the President will ask USDA to consider beginning the process of revising the Thrifty Food Plan to better reflect the modern cost of a healthy basic diet.223
Ensure Equitable and Effective Delivery of Direct Payments. As the President fights to get Americans the full $2,000 in direct payments they deserve, his administration is also working to ensure that all those who are eligible receive their full payments. Many Americans faced challenges receiving the first round of direct payments and as many as eight million eligible households did not receive the payments issued in March. In December, Congress passed legislation that would provide Americans with $600 in stimulus. The President’s American Rescue Plan proposes an additional $1,400 per-person payments to ensure that households get the support they need to help pay bills, put food on the table, and support small businesses and their communities. While Treasury and career staff at the IRS have worked tirelessly to deliver two rounds of payments in the midst of a pandemic, the work is far from over. To ensure equitable and effective delivery of direct payments and focus on getting relief to eligible individuals who have not received the financial assistance to which they are entitled, the President is asking the Department of Treasury to consider taking a series of actions to expand and improve delivery of Economic Impact Payments including establishing online tools for claiming their payments, working to make sure that those who have not yet accessed their funds get the relief they deserve, and analyzing unserved households to inform additional outreach efforts.224
Guarantee that No American Has to Choose Between Paying Their Bills and Keeping Themselves and Their Families Safe from COVID-19. In 2019, 43% of American households reported having at least one member with pre-existing conditions, many of whom may have a heightened risk of serious illness or death if they contract COVID. President Biden believes that workers should have the right to safe work environments and that no one should have to choose between their livelihoods and their own or their families’ health. As one of many measures to help keep workers and their families’ safe throughout the pandemic, the President is asking the Department of Labor to consider clarifying that workers have a federally guaranteed right to refuse employment that will jeopardize their health and if they do so, they will still qualify for unemployment insurance.225
Help Families, Workers and Small Businesses Access Relief Resources Quickly, Easily and Equitably through Coordinated Benefit Delivery Teams. During the pandemic government programs have provided much needed support to help tens of millions of Americans pay rent, mortgages and other bills, get the food they need, and access healthcare. However, critical support does not always reach the people who need it: families struggle to navigate complicated eligibility rules while over 20% of Earned Income Tax Credits go unclaimed; many small businesses in communities of color cannot easily access loans; and according to one survey less than 40% of service workers who were laid off or furloughed at the height of pandemic closures actually received timely unemployment benefits due to system failures as applications surged. At the same time, an estimated 47% of children live in households that have trouble covering usual expenses such as food, housing, and medical care. The stakes are too high and too many families are in need for people not to get the relief that they are entitled to. The Biden-Harris Administration is establishing a network of benefit delivery teams and a coordination structure across federal and state administered programs to reduce the time and burden to access urgent support that provides greater stability and builds towards an equitable recovery.226
The federal government should only award contracts to employers who give their workers the pay and benefits they have earned; President Biden is today directing his administration to start the work that would allow him to issue an Executive Order within the first 100 days that requires federal contractors to pay a $15 minimum wage and provide emergency paid leave to workers.227
He is also taking critical steps to protect and empower federal employees, who dedicate their careers to serving the American people. They keep us healthy, safe, and informed, and their work transcends partisan politics. They are health care workers who care for veterans, the elderly, and the disabled. They are expert scientists, medical doctors, and technicians who maintain world-class standards, prevent and combat the spread of infectious diseases, and save countless lives. They deliver our mail, run our national parks, keep our federal buildings up and running, help protect us against climate change and environmental poisoning, and ensure that the law is applied faithfully and fairly. They are talented, hard-working, and inspiring Americans, worthy of the utmost dignity and respect. But, over the last four years, they’ve been undermined and demoralized. The President will sign an executive order taking steps to protect and empower federal employees who are so essential to this country. It:
- Restores collective bargaining power and worker protections by revoking Trump Executive Orders 13836, 13837, and 13839. It goes further to direct agencies to bargain over permissible, non-mandatory subjects of bargaining when contracts are up for negotiation so that workers have a greater voice in their working conditions.
- Eliminates Schedule F, which undermines the foundations of the civil service. Its existence threatens the critical protections of career employees and provides a pathway to burrow political appointees into the civil service.
- Promotes a $15 minimum wage. The Executive Order directs the Office of Personnel Management to develop recommendations to pay more federal employees at least $15 per hour.
These steps will help ensure the federal government is a model employer and restore protections to career civil servants who are so essential to this country.228
[Earlier in February 2021], the House and Senate approved a fiscal 2021 budget resolution (S. Con. Res. 5), directing committees to use the budget reconciliation process to draft COVID-19 relief legislation consistent with President Joe Biden’s $1.9 trillion American Rescue Plan. The budget reconciliation process allows the Senate to advance spending and tax related legislation with a simple majority vote instead of the 60-vote threshold needed for most legislation.229
During the week of February 8, a number of House committees advanced portions of the President’s plan along party lines. The bills include proposals to extend enhanced unemployment benefits, provide $1,400 stimulus checks, increase Affordable Care Act (ACA) tax credits, provide subsidies for COBRA coverage, increase funding for Medicaid and the Children’s Health Insurance Program (CHIP), extend and modify tax credits for paid sick and family leave and employee retention, and increase the federal minimum wage to $15 per hour by 2025.230
The relief package is expected to include nearly $1.9 trillion in emergency funding, including:
- $350 billion for state, tribal, and local governments;
- $220 billion for education and community support programs;
- $95.5 billion for transportation and infrastructure programs;
- $92.2 billion for public health programs;
- $75 billion for housing and community development programs;
- $50 billion for small business relief;
- $13.5 billion for veterans health care; and
- $10.4 billion for food and nutrition support programs.
The House Budget Committee and Rules Committee will meet in the coming days to combine the bills into one reconciliation package and set rules for consideration. House Majority Leader Steny Hoyer (D-MD) informed lawmakers that the House could vote on the package as early as Friday, February 26.231
Senate leaders are expected to skip the committee drafting process and bring the House package directly to the floor the first week of March. Since the budget reconciliation process has limitations, House and Senate leaders have been consulting the Senate parliamentarian throughout this process to determine which provisions can remain in a final package. The Senate will likely amend the package before approving it, requiring the House to vote again. Congressional Democratic leaders aim to pass the stimulus package and send it to the President before March 15.232
Although House and Senate Democratic leaders have said they want to pass a minimum wage increase, several Democratic Senators have expressed reservations with its inclusion in a COVID-19 relief package. Additionally, President Biden has acknowledged that the wage provisions may need to be removed and considered separately due to parliamentary rules that govern consideration of budget reconciliation measures.233
[Meanwhile] [s]ome Senate Democrats are urging President Joe Biden to overhaul the nation’s approach to handing out stimulus payments, the direct financial relief deployed by Congress to help millions of Americans affected by the economic collapse that followed the coronavirus pandemic.234
Instead of providing discrete rounds of stimulus checks that are negotiated each time and arrive months apart, the federal government should provide recurring checks to help families get by until COVID-19 is over, the 10 lawmakers said in a letter released on Tuesday.235
The request comes as the Senate takes up Mr. Biden’s $1.9 trillion relief package this week, which would include a third round of stimulus checks that would direct $1,400 to millions of eligible Americans. Congress distributed $1,200 checks a year ago under the Coronavirus Aid, Relief and Economic Security (CARES) Act, and sent an additional $600 payment in December as part of a broader stimulus bill. The senators didn’t specify an amount they are seeking for monthly direct aid.236
The idea of issuing recurring stimulus payments as a way to speed up the economic recovery has been championed by progressives and some Democrats. In January, more than 50 House members urged the Biden administration to back a proposal for $2,000 monthly payments until the pandemic ends. Supporters of the idea point out that financial hardship remains widespread around the U.S. almost a full year after COVID-19 effectively shuttered the economy. Despite the ongoing recovery, a third of adults are struggling to pay their bills, while employers have slashed roughly 10 million jobs from their payrolls during the crisis, according to an analysis by the Center on Budget and Policy Priorities.237
“The decades of research on stimulus checks back up the argument that much of this is spent, which helps stimulate the economy, and sources like the Census show there is immense need” for more funding despite the fitful recovery, said Claudia Sahm, an economist who has worked at the Federal Reserve and the Washington Center for Equitable Growth. She added, “The reason we aren’t in the 1933 world when things were really bad is because the federal government and Federal Reserve stepped in — but we aren’t out of the woods yet.”238
The senators who signed the letter, including Bernie Sanders of Vermont, Elizabeth Warren of Massachusetts and Ron Wyden of Oregon, argue that aid such as enhanced unemployment benefits hasn’t reached every family hurt by the economic crunch. Millions “do not qualify for unemployment insurance after seeing their hours reduced, switching to lower-paying jobs, or temporarily leaving the workforce to care for family members during the pandemic,” they wrote in the letter. “Direct payments are crucial for supporting struggling families who aren’t reached by unemployment insurance.”239
According to Sam Thielman, [o]nce again, the United States finds itself in the middle of a huge economic disaster that can only be addressed by monkeying with fiscal policy, and you know what that means: People who already have lots of money think the government ought to be very careful not to spend too much helping the poor.240
The current debate centers on the Biden administration’s coronavirus stimulus plan, the centerpiece of which — as far as most Americans have been concerned — is a $2,000 stimulus check for Americans making under $75,000 per year, which Democrats had promised in December if they took control of the Senate after Senate Republicans blocked a bill approving the payments. (A stimulus of $600 got sent out under former President Donald Trump and former Senate Majority Leader Mitch McConnell, R-Ky., as part of a failed effort to retain control of the Senate by winning runoff elections in Georgia.)241
Now that check will be $1,400 — Democrats say the $600 from the end of the year counts against the total $2,000 — and Democrats are entertaining the idea of lowering the threshold at which even that paltry one-time sum gets phased out.242
But the only way to build a working society that isn’t simply a playground for the rich at the expense of the poor is to make public benefits truly public; taking stimulus payments or health care should be as boring and rote as mailing a letter. Otherwise we’re all on our way to a new Gilded Age, where instead of a public post office, all we will have is Amazon (quietly surveilling our neighborhoods and selling the footage to the police); where instead of a government taxing us to provide public goods, corporations can and will impose taxes for their benefit; and where, instead of health care for all, private euity owns a controlling interest in all the hospitals and profits off of our poor health. We can have that, or we can have civilization.243
We can wring our hands at the idea that someone, somewhere, might get a little too much stimulus and deny many people the money they need, or, as Biden said — and to which we must hold him — we can learn from a past when we did far too little and suffered for it.244
Conclusion
One of the deep and great lessons of the coronavirus pandemic is that countries may or shall not live alone by the mighty powers of their militaries but by their ability to rise up to challenges as they fall on their laps or rear their ugly monstrous heads from time to time such as the coronavirus pandemic and resolve them. That is one the great lessons taught by the Mekong-Pacific Knights’ countries which rose early enough like Poseidons from the depth of the Asia-Pacific Seas and were able to crushingly defeat the pandemic not by their military might (kinetic power) but by “smart powers” (non-kinetic power) before the pandemic could do any substantial damage to their countries. In contrast, a large number of the greatest military powers on earth can be seen to have been humbled and hobbled by the pandemic, running from pillars to poles, stumbling and falling along, to face the existential challenge posed by the pandemic.
A virus, invisible to the eyes, and that cannot be grabbed or held by the palm of our hands came like a thunderbolt from Wuhan city, China, allowed to spread all over the world through human vectors (air travellers), to send human beings over the biological cliff, tear up the socioeconomic fabrics of societies, undermine national and international security, and caused untold human sufferings. Governments all over the world can be seen panicking, running helter-skelter like ponderous elephants pursued by hungry predators.
The United States went to South China Sea to challenge and intimidate China for the better part of 2020 with their awesome naval ships and other war machines but came back with no “war booties”.
When lockdowns, other form of restrictions, face mask wearing, keeping of social distances, and other safety measures could not protect and deliver the world from the ravages of the pandemic, attention has now shifted to production and administration of vaccines to help halt the spread of the pandemic. But no one is really sure whether this would be the case in the final analysis as the governments, scientific community, and medical sector have not been able to answer certain fundamental and germane questions about the efficacy of the vaccines now on the global market shelves: After vaccination, can one stop wearing face masks, bridge social distancing?; Would vaccination means return to the Old Normal?; Does vaccination actually offer resistance to Covid-19?; Would vaccination stop transmission, protection against contagion or infection?; What is the duration of the effect of the vaccines in human bodies?; After vaccination, can we kiss and hug each other again?; What really is the benefit of getting vaccinated when no one is certain of its overall efficacy?
Vaccines and vaccination have long history on their own. They are part and parcel of modernization and post-modernism especially in the health sector. There are no doubts that they can be reliable and indeed have helped saved a large proportion of world population from vicious and voracious epidemics, pandemics, and other forms of diseases as long as we can all remember. They are used both by soldiers and civilians. But vaccines are not military invention or instrument, thus does not possess kinetic power. Vaccines may not also be classified as either soft or smart power but may be regarded as hybrid power.
But there is also the politics of vaccines and vaccination itself. First is the geopolitics by which one can see clear that China has lost the battle already even with its vaccine international diplomacy. Its main archrival in Asia, India, is also producing its own vaccines and may not be dependent on importation of vaccines from China at all. So also is its main global archrival, the United States, that will definitely not touch any Chinese vaccine with a long pole because it is producing and already administering its own various types of vaccines. Most European countries, because of their geopolitical animus with China, are exclusively relying on vaccines produced in Europe and the United States. China, Russia and India including the Euro-American vaccines are being seen targeted at the middle- and low-income earning countries.
The second is that vaccine production, marketing and distribution is a Big Business, and nothing more. Not all countries can produce vaccines. Only few can do so. Vaccine production is a business-to-business process. It is hardly a philantropic engagement. Governments must pay for whatever vaccine they are importing to their countries. This cost money especially when situated within the each country’s currency exchange rates. However, it is business-to-government-to-public enterprise, and one is as important as the other.